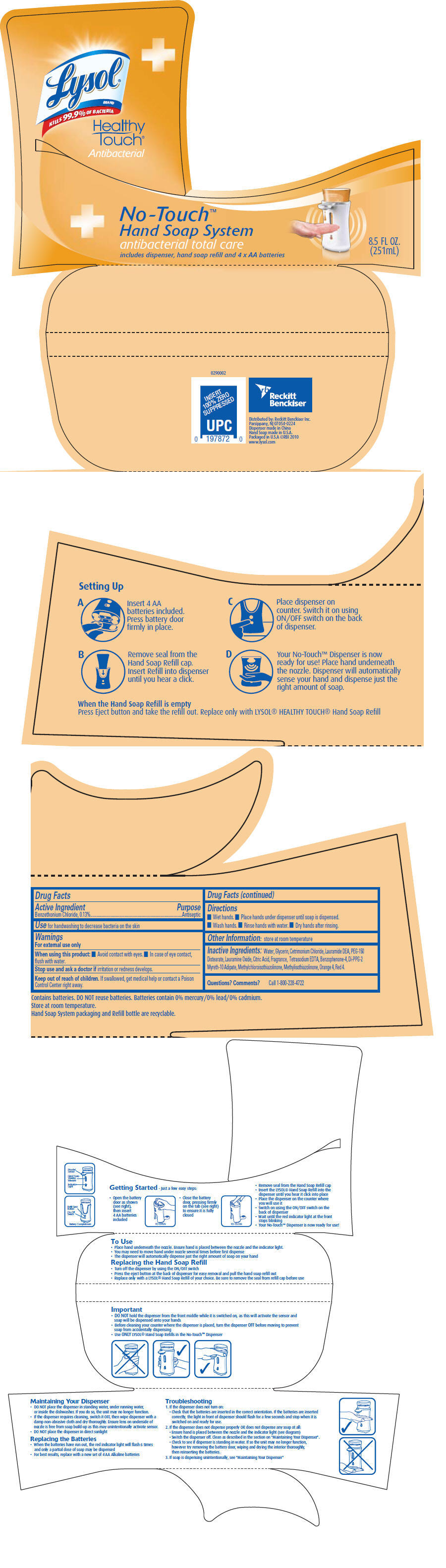
LYSOL HEALTHY TOUCH- benzethonium chloride solution
RB Health (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lysol
®Healthy Touch
®Antibacterial
No-Touch Hand Soap System
Directions
- Wet hands.
- Place hands under dispenser until soap is dispensed.
- Wash hands.
- Rinse hands with water.
- Dry hands after rinsing.
| LYSOL HEALTHY TOUCH
benzethonium chloride solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |
Revised: 9/2023
Document Id: 04b8510e-c9c7-76de-e063-6394a90ad0b4
Set id: d1311754-5f7c-4ead-9c36-343dfcc7a604
Version: 4
Effective Time: 20230906
RB Health (US) LLC