XOFLUZA- baloxavir marboxil powder
Evonik Corporation
----------
Baloxavir marboxil, S-033188 (DOP-13)
Drum Label, Baloxavir marboxil
Caution: For manufacturing, processing or repacking. Rx only.
EV022X
S-033188 API Milled
Baloxavir Marboxil (DOP13)
Caution: LIver, Potent, Suspect Reproductive
Storage conditions: 1 - 30 C
Batch number:
Manufacture Date:
Manufactured and tested by Evonik Corporation, Tippecanoe Laboratories, 1650 Lilly Road, Lafayette, IN 47909

Warehouse put-away label, net weight
Product is weighed in the warehouse and the weight is on this label. Label also includes the storage location (in the warehouse) and the SAP transfer order#.
Material number : EV022X
Material description:
Batch:
TO:
SU: storage unit
Quantity: x.xx Kg
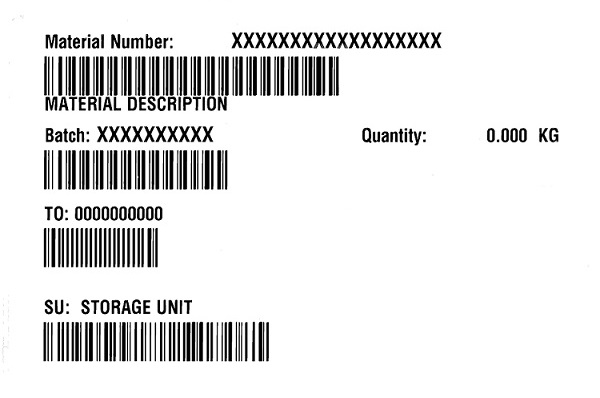
May 2022 Warehouse KG label
S-033188 API Milled
Put-Away Label
Material Number 99144221
Storage Unit
Net Weight xx Kg
Lot/Batch
Old Material Number EV022X
QR code box
Temperature Conditions: 1 to 30°C
Hazards:
Liver Potent Suspect Reproductive
Health (U) Unknown
Fire (U) Unknown
Reactivity (U) Unknown
Special P (highly potent)
Evonik Corporation
1650 Lilly Road Lafayette, Indiana 47909 USA
Phone: 765-477-4300 Fax: 765-477-4291
Evonik: Leading beyond Chemistry
Emergency Information: Chemtrec 800-424-9300

| XOFLUZA
baloxavir marboxil powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Evonik Corporation (130890994) |