DOMEBORO- aluminum acetate gel
Advantice Health, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DOMEBORO® GEL
Uses
temporarily relieves minor skin irritations due to:
- poison ivy
- poison oak
- poison sumac
- insect bite
- athlete's foot
- rashes caused by soaps, detergents, cosmetics, or jewelry
Inactive ingredients
Boric Acid, Ethanol, Glycerin, Hydrochloric Acid, Hypromellose, Menthol, Polysorbate 20, Propylene Glycol, Purified Water
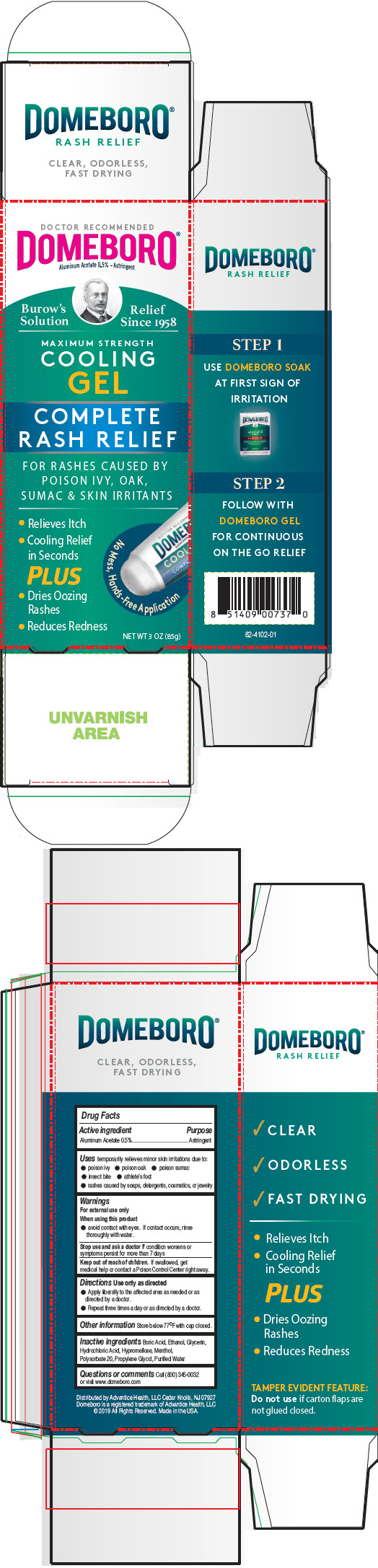
PRINCIPAL DISPLAY PANEL - 85 g Tube Carton
DOCTOR RECOMMENDED
DOMEBORO®
Aluminum Acetate 0.5% - Astringent
Burrow's
Solution
Relief
Since 1958
MAXIMUM STRENGTH
COOLING
GEL
COMPLETE
RASH RELIEF
FOR RASHES CAUSED BY
POISON IVY, OAK,
SUMAC & SKIN IRRITANTS
- Relieves Itch
- Cooling Relief
in Seconds
PLUS - Dries Oozing
Rashes - Reduces Redness
No Mess, Hands-Free Application
NET WT 3 OZ (85g)
| DOMEBORO
aluminum acetate gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Advantice Health, LLC. (192527062) |
Revised: 9/2021
Document Id: 4bf19792-5061-4f1f-9034-192e5e77301e
Set id: cfd8fc64-79a4-4a49-b3eb-d40e644cde7b
Version: 2
Effective Time: 20210916
Advantice Health, LLC.