HEPARIN SODIUM AND SODIUM CHLORIDE- heparin sodium injection, solution
Baxter Healthcare Corporation
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
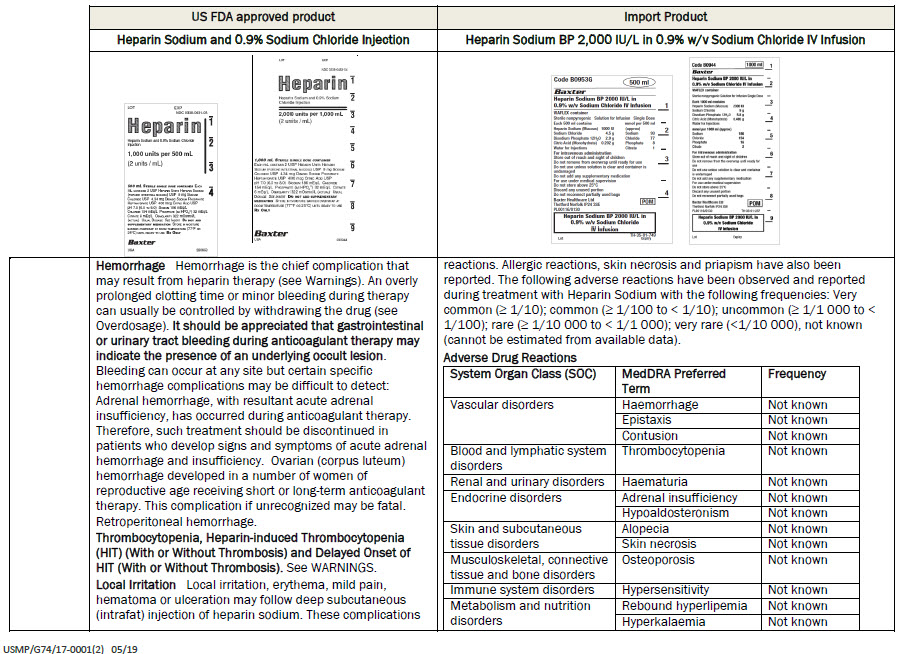
Heparin Sodium bp 2000 iu/l in
0.9% w/v sodium chloride iv infusion
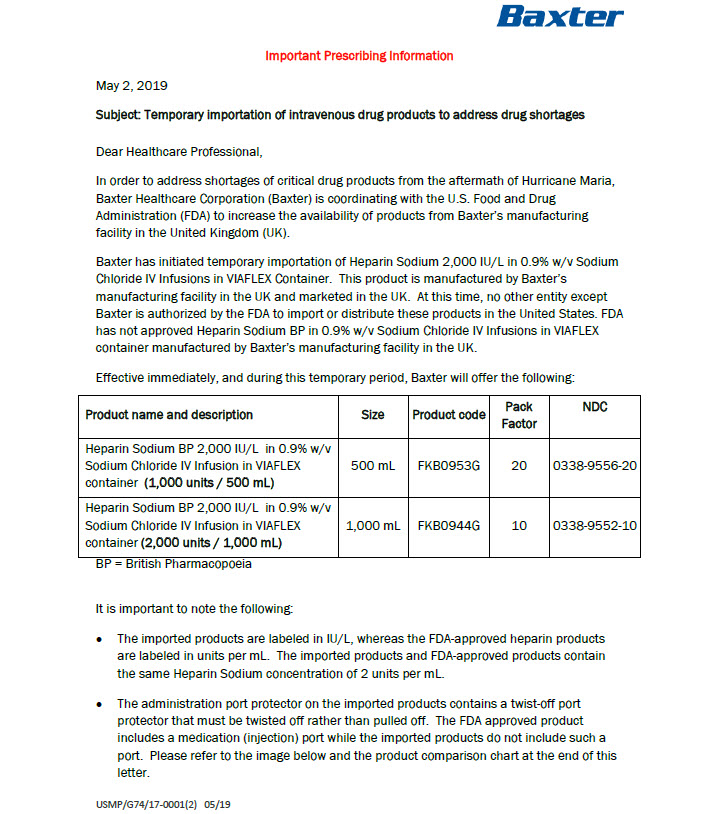
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
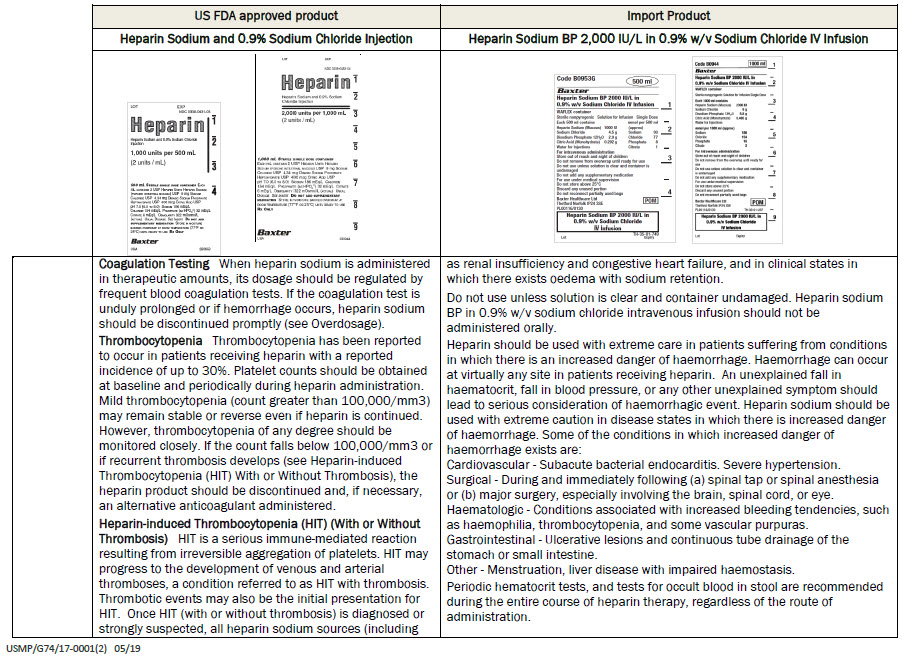
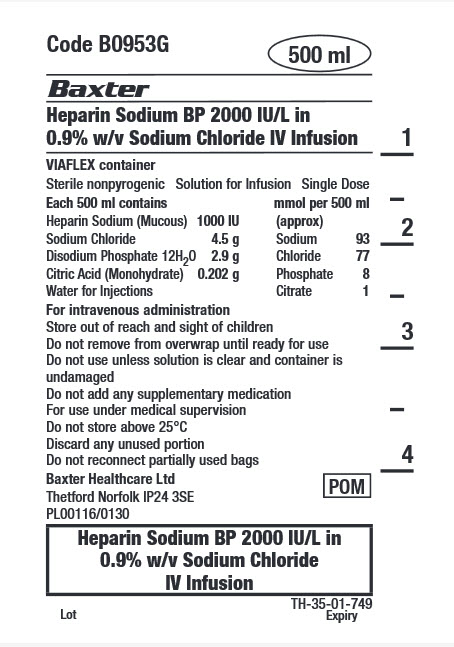
Code B0953G
500 ml
Baxter Logo
Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride IV Infusion
VIAFLEX container
Sterile nonpyrogenic
Solution for Infusion
Single Dose
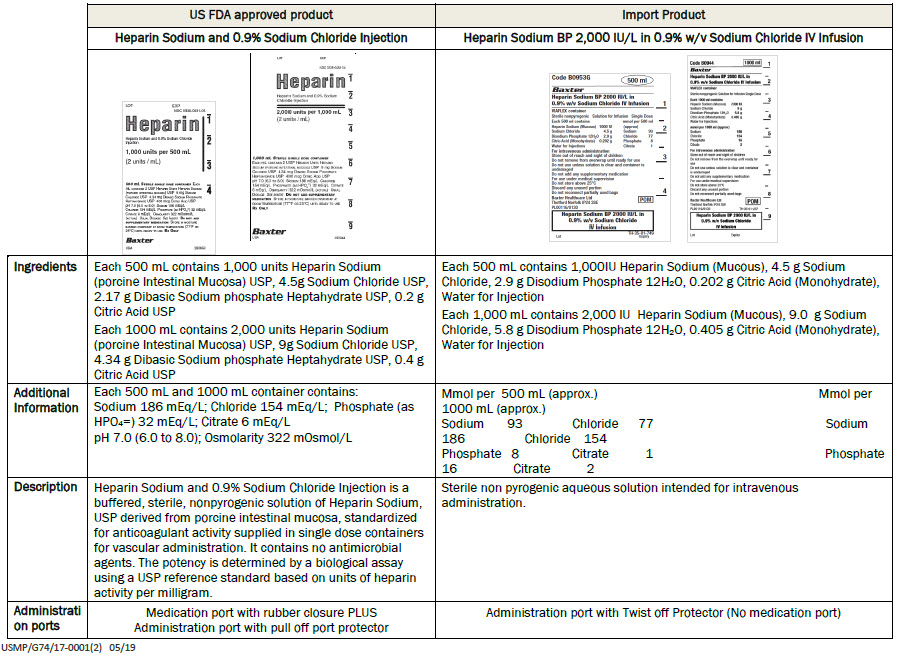
Each 500 ml contains
Heparin Sodium (Mucous) 1000 IU
Sodium Chloride 4.5 g
Disodium Phosphate 12H 2 0 2.9 g
Citric Acid (Monohydrate) 0.202g
Water for Injections
mmol per 500 ml (approx)
Sodium 93
Chloride 77
Phosphate 8
Citrate 1
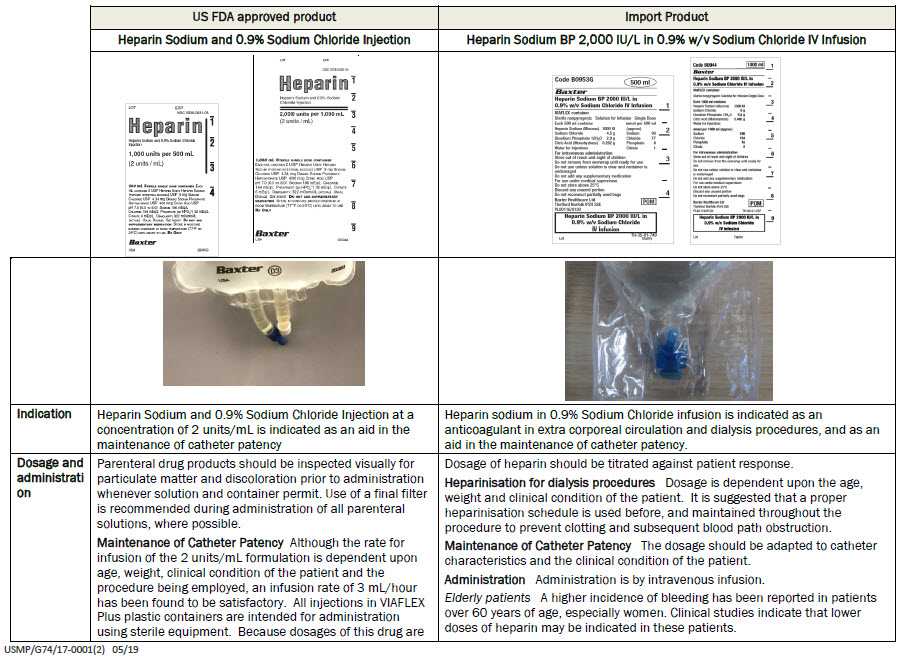
For intravenous administration
Store out of reach and sight of children
Do not remove from overwrap until ready for use
Do not use unless solution is clear and container is
undamaged
Do not add any supplementary medication
For use under medical supervision
Do not store above 25°C
Discard any unused portion
Do not reconnect partially used bags
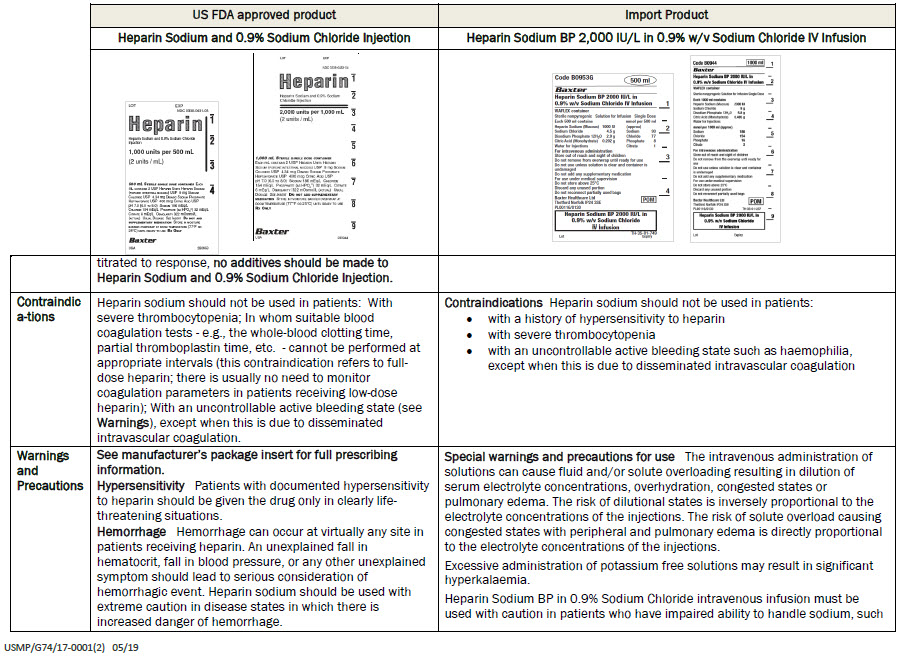
Baxter Healthcare Ltd
Thetford Norfolk IP24 3SE
PL00116/0130
POM symbol
Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride
IV Infusion
TH-35-01-749
Lot
Expiry
1
2
3
4
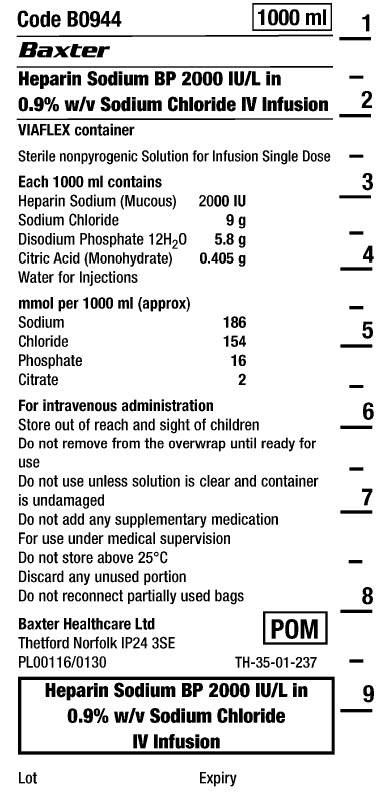
Code B0944
1000 ml
Baxter Logo
Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride IV Infusion
VIAFLEX container
Sterile nonpyrogenic
Solution for Infusion
Single Dose
-
Each 1000 ml contains
Heparin Sodium (Mucous) 2000 IU
Sodium Chloride 9 g
Disodium Phosphate 12H 2 0 5.8 g
Citric Acid (Monohydrate) 0.405 g
Water for Injections
mmol per 1000 ml (approx)
Sodium 186
Chloride 154
Phosphate 16
Citrate 2
For intravenous administration
Store out of reach and sight of children
Do not remove from overwrap until ready for
use
Do not use unless solution is clear and container
is undamaged
Do not add any supplementary medication
For use under medical supervision
Do not store above 25°C
Discard any unused portion
Do not reconnect partially used bags
Baxter Healthcare Ltd
Thetford Norfolk IP24 3SE
PL00116/0130
POM symbol
TH-35-01-237
Heparin Sodium BP 2000 IU/L in
0.9% w/v Sodium Chloride
IV Infusion
Lot
Expiry
1
2
3
4
5
6
7
8
9
| HEPARIN SODIUM AND SODIUM CHLORIDE
heparin sodium injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| HEPARIN SODIUM AND SODIUM CHLORIDE
heparin sodium injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Corporation (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Ltd | 221478644 | ANALYSIS(0338-9556, 0338-9552) , MANUFACTURE(0338-9556, 0338-9552) , LABEL(0338-9556, 0338-9552) , PACK(0338-9556, 0338-9552) , STERILIZE(0338-9556, 0338-9552) | |