Label: UPNEEQ- oxymetazoline hydrochloride ophthalmic solution/ drops
-
NDC Code(s):
73687-062-25,
73687-062-32,
73687-062-34,
73687-062-45, view more73687-062-68, 73687-062-70, 73687-062-80
- Packager: RVL Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 9, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use UPNEEQ® safely and effectively. See full prescribing information for UPNEEQ.
UPNEEQ (oxymetazoline hydrochloride ophthalmic solution), 0.1%, for topical ophthalmic use
Initial U.S. Approval: 1964INDICATIONS AND USAGE
UPNEEQ is indicated for the treatment of acquired blepharoptosis in adults. (1)
DOSAGE AND ADMINISTRATION
Instill one drop into one or both ptotic eye(s) once daily. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution, 0.1% oxymetazoline as salt, equivalent to 0.09% oxymetazoline as base. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Ptosis may be associated with neurologic or orbital diseases such as stroke and/or cerebral aneurysm, Horner syndrome, myasthenia gravis, external ophthalmoplegia, orbital infection and orbital masses. Consideration should be given to these conditions in the presence of ptosis with decreased levator muscle function and/or other neurologic signs. (5.1)
- Alpha-adrenergic agonists as a class may impact blood pressure. Advise patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens. (5.2)
- Use with caution in patients with cerebral or coronary insufficiency or Sjögren’s syndrome and advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop. (5.3)
- Advise patients to seek immediate medical care if pain, redness, blurred vision and photophobia occur (signs and symptoms of acute angle closure). (5.4)
ADVERSE REACTIONS
Most common adverse reactions (incidence 1-5%) are: punctate keratitis, conjunctival hyperemia, dry eye, vision blurred, instillation site pain, eye irritation and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact RVL Pharmaceuticals, Inc. at 1-877-482-3788 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Ptosis as Presenting Sign of Serious Neurologic Disease
5.2 Potential Impacts on Cardiovascular Disease
5.3 Potentiation of Vascular Insufficiency
5.4 Risk of Angle Closure Glaucoma
5.5 Risk of Contamination
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Anti-hypertensives/Cardiac Glycosides
7.2 Monoamine Oxidase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Instill one drop of UPNEEQ into one or both ptotic eye(s) once daily. Discard the single patient-use container immediately after dosing.
Contact lenses should be removed prior to instillation of UPNEEQ and may be reinserted 15 minutes following its administration.
If more than one topical ophthalmic drug is being used, the drugs should be administered at least 15 minutes between applications.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Ptosis as Presenting Sign of Serious Neurologic Disease
Ptosis may be associated with neurologic or orbital diseases such as stroke and/or cerebral aneurysm, Horner syndrome, myasthenia gravis, external ophthalmoplegia, orbital infection and orbital masses. Consideration should be given to these conditions in the presence of ptosis with decreased levator muscle function and/or other neurologic signs.
5.2 Potential Impacts on Cardiovascular Disease
Alpha-adrenergic agonists may impact blood pressure. UPNEEQ should be used with caution in patients with severe or unstable cardiovascular disease, orthostatic hypotension, and uncontrolled hypertension or hypotension. Advise patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension/hypotension to seek immediate medical care if their condition worsens.
5.3 Potentiation of Vascular Insufficiency
UPNEEQ should be used with caution in patients with cerebral or coronary insufficiency, or Sjögren’s syndrome. Advise patients to seek immediate medical care if signs and symptoms of potentiation of vascular insufficiency develop.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 360 subjects with acquired blepharoptosis were treated with UPNEEQ once daily in each eye for at least 6 weeks in three controlled Phase 3 clinical trials, including 203 subjects treated with UPNEEQ for 6 weeks and 157 subjects treated with UPNEEQ for 12 weeks. Adverse reactions that occurred in 1-5% of subjects treated with UPNEEQ were punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation and headache.
-
7 DRUG INTERACTIONS
7.1 Anti-hypertensives/Cardiac Glycosides
Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as beta-blockers, anti-hypertensives, and/or cardiac glycosides is advised.
Caution should also be exercised in patients receiving alpha adrenergic receptor antagonists such as in the treatment of cardiovascular disease, or benign prostatic hypertrophy.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on UPNEEQ use in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. In animal reproduction studies, there were no adverse developmental effects observed after oral administration of oxymetazoline hydrochloride in pregnant rats and rabbits at systemic exposures up to 7 and 278 times the maximum recommended human ophthalmic dose (MRHOD), respectively, based on dose comparison. [see Data]. The estimated background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.Animal Data
Effects on embryo-fetal development were evaluated in rats and rabbits following oral administration of oxymetazoline hydrochloride during the period of organogenesis. Oxymetazoline hydrochloride did not cause adverse effects to the fetus at oral doses up to 0.2 mg/kg/day in pregnant rats during the period of organogenesis (28 times the MRHOD, on a dose comparison basis). Oxymetazoline hydrochloride did not cause adverse effects to the fetus at oral doses up to 1 mg/kg/day in pregnant rabbits during the period of organogenesis (278 times the MRHOD, on a dose comparison basis). Maternal toxicity, including decreased maternal body weight, was produced at the high dose of 1 mg/kg/day in pregnant rabbits and was associated with findings of delayed skeletal ossification.In a rat prenatal and postnatal development study, oxymetazoline hydrochloride was orally administered to pregnant rats once daily from gestation day 6 through lactation day 20. Maternal toxicity was produced at the high dose of 0.2 mg/kg/day (28 times the MRHOD, on a dose comparison basis) in pregnant rats and was associated with an increase in pup mortality and reduced pup body weights. Delayed sexual maturation was noted at 0.1 mg/kg/day (14 times the MRHOD, on a dose comparison basis). Oxymetazoline hydrochloride did not have any adverse effects on fetal development at a dose of 0.05 mg/kg/day (7 times the MRHOD, on a dose comparison basis).
8.2 Lactation
Risk Summary
No clinical data are available to assess the effects of oxymetazoline on the quantity or rate of breastmilk production, or to establish the level of oxymetazoline present in human breastmilk post-dose. Oxymetazoline was detected in the milk of lactating rats. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for UPNEEQ and any potential adverse effects on the breastfed child from UPNEEQ. -
10 OVERDOSAGE
Accidental oral ingestion of topical intended solutions (including ophthalmic solutions and nasal sprays) containing imidazoline derivatives (e.g., oxymetazoline) in children has resulted in serious adverse events requiring hospitalization, including nausea, vomiting, lethargy, tachycardia, decreased respiration, bradycardia, hypotension, hypertension, sedation, somnolence, mydriasis, stupor, hypothermia, drooling, and coma. Keep UPNEEQ out of reach of children.
-
11 DESCRIPTION
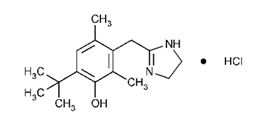
UPNEEQ (oxymetazoline hydrochloride ophthalmic solution), 0.1% contains oxymetazoline hydrochloride, an alpha adrenoceptor agonist. UPNEEQ is an aseptically prepared, sterile, non-preserved ophthalmic solution. The chemical name is 6-tert-Butyl-3-(2-imidazolin-2-ylmethyl)-2,4- dimethylphenol monohydrochloride, and the molecular mass is 296.84. Oxymetazoline HCl is freely soluble in water and ethanol and has a partition coefficient of 0.1 in 1-octanol/water. The molecular formula of oxymetazoline HCl is C16H24N2O∙HCl, and its structural formula is:
Each mL of UPNEEQ (oxymetazoline hydrochloride ophthalmic solution) 0.1% contains 1 mg of oxymetazoline hydrochloride, equivalent to 0.9 mg (0.09%) of oxymetazoline free base. The ophthalmic solution contains the following inactive ingredients: calcium chloride, hydrochloric acid (used to adjust pH to 5.8 to 6.8), hypromellose, magnesium chloride, potassium chloride, sodium acetate, sodium chloride, sodium citrate, and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Oxymetazoline is an alpha adrenoceptor agonist targeting a subset of adrenoreceptors in Mueller’s muscle of the eyelid.
12.2 Pharmacodynamics
In safety and efficacy studies, Study RVL-1201-201 and Study RVL-1201-202, the pharmacodynamics of UPNEEQ was assessed using an objective photographic measure, marginal reflex distance 1 (MRD1). MRD1 is the distance from the central pupillary light reflex to the central margin of the upper lid. The maximum increase in MRD1 was observed approximately 2 hours post dose in both studies. The MRD1 increase continued through 8 hours post dose.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of oxymetazoline was evaluated in 24 healthy adult subjects following single-drop administration of UPNEEQ to each eye. The total dose of oxymetazoline HCl was 0.07 mg. Following ocular administration of UPNEEQ, the time to reach peak oxymetazoline concentration (Tmax) values ranged from 0.5 to 12 hours with a median Tmax of 2 hours. The oxymetazoline mean ± standard deviation (SD) peak concentration (Cmax) and area under the concentration-time curve (AUCinf) were 30.5 ± 12.7 pg/mL and 468 ±214 pg*hr/mL, respectively.Distribution
An in vitro study demonstrated that oxymetazoline is 56.7% to 57.5% bound to human plasma proteins.Elimination
The oxymetazoline mean terminal half-life (t½) following administration of UPNEEQ in healthy adult subjects is 8.3 hours and ranged from 5.6 to 13.9 hours.
Metabolism
In vitro studies using human liver microsomes showed that oxymetazoline was minimally metabolized, generating mono-oxygenated and dehydrogenated products of oxymetazoline. The percentage of parent drug oxymetazoline remaining was 95.9% after a 120-minute incubation with human liver microsomes.Excretion
The excretion of oxymetazoline following administration of UPNEEQ has not been characterized in humans. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Oxymetazoline hydrochloride was not associated with an increased incidence of neoplastic or proliferative changes in transgenic mice given oral doses of 0.5, 1.0, or 2.5 mg/kg/day oxymetazoline hydrochloride for 6 months.Mutagenesis
Oxymetazoline hydrochloride revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and human lymphocyte chromosomal aberration assay) and one in vivo gentoxicity test (mouse micronucleus assay).Impairment of Fertility
Effects on fertility and early embryonic development were evaluated in rats following oral administration of 0.05, 0.1, or 0.2 mg/kg/day oxymetazoline hydrochloride prior to and during mating and through early pregnancy. Decreased number of corpora lutea and increased post-implantation losses were noted at 0.2 mg/kg/day oxymetazoline hydrochloride (28 times the MRHOD, on a dose comparison basis). However, no treatment related effects on mating parameters were noted at 0.2 mg/kg/day oxymetazoline hydrochloride. -
14 CLINICAL STUDIES
UPNEEQ was evaluated for the treatment of acquired blepharoptosis in two randomized, double-masked, vehicle-controlled, parallel-group clinical efficacy trials. Both studies were randomized in an approximate 2:1 ratio of active versus vehicle. Efficacy was assessed with the Leicester Peripheral Field Test (LPFT) (primary) and photographic measurement of Marginal reflex distance 1 (MRD1). The primary efficacy endpoints were ordered in a hierarchy to compare UPNEEQ to vehicle on the mean increase from baseline (Day 1 Hour 0) in number of points seen on the top 4 rows of the LPFT in the study eye at Hour 6 on Day 1 and Hour 2 on Day 14.
In Trial 1, a total of 140 subjects were randomized 94 patients to the UPNEEQ group and 46 patients to the vehicle group. Treatments were administered once daily to each eye for 42 days (6 weeks). The mean age of the subjects was 64 years. In Trial 2, a total of 164 subjects were randomized 109 patients to the UPNEEQ group and 55 patients to the vehicle group. Treatments were administered once daily to each eye for 42 days (6 weeks). The mean age of the subjects was 63 years.
In both trials, each patient had a designated study eye. The increases in the number of points seen in the superior visual field in the study eye of the UPNEEQ group compared to the vehicle group were statistically significant at both time points, showing that the improvement in superior visual field was evident at the 2-hour time point and maintained at the 6-hour time point. The results from both trials on the primary endpoint are presented below.Observed and Change from Baseline in Mean Points Seen in Superior Visual Field on LPFT in the Study Eye at Primary Efficacy Time Points (ITT Population)
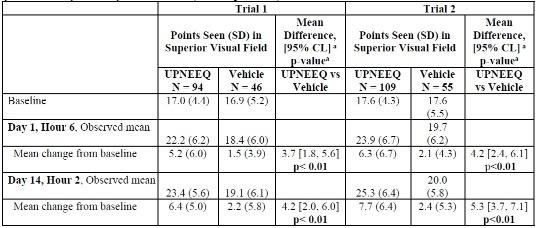
CL = confidence limit; LPFT = Leicester Peripheral Field Test; ITT (intent-to-treat) – included all randomized subjects
who received at least one dose of study medication; SD = standard deviation
a p-value and 95% CI were based on ANCOVA model adjusted for baseline LFPT points.Marginal reflex distance 1 (MRD1), showed a positive effect with UPNEEQ treatment. Greater MRD1 increases were observed for the UPNEEQ group than the vehicle group on day 1 at 6 hours post dose and on day 14 at 2 hours post dose.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
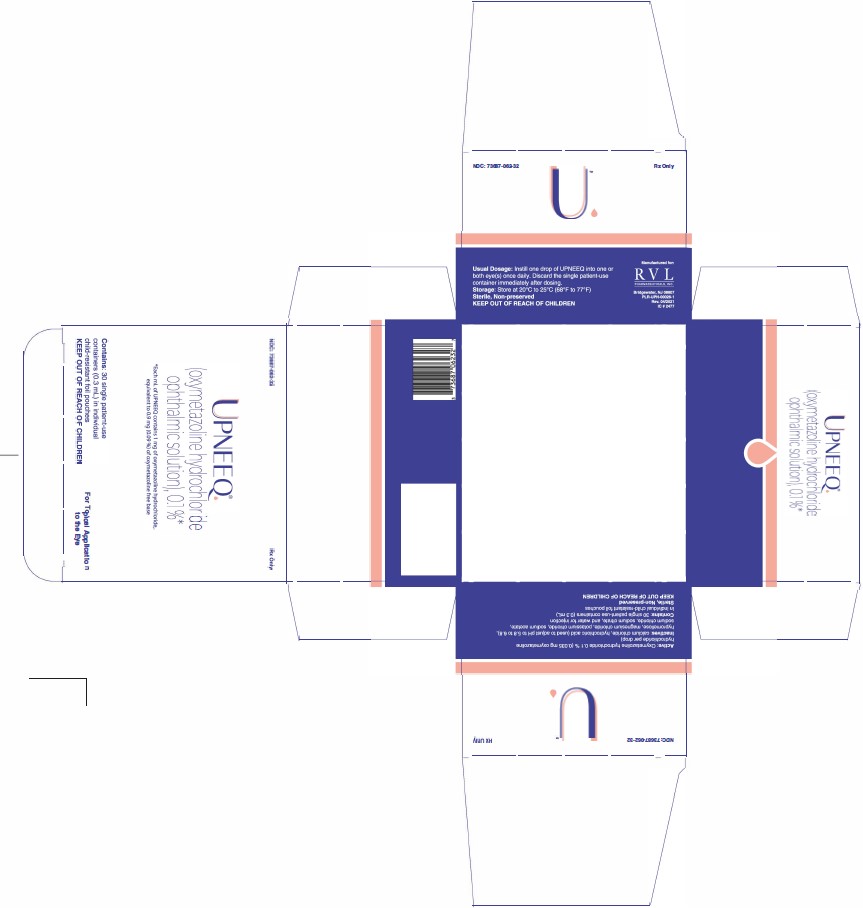
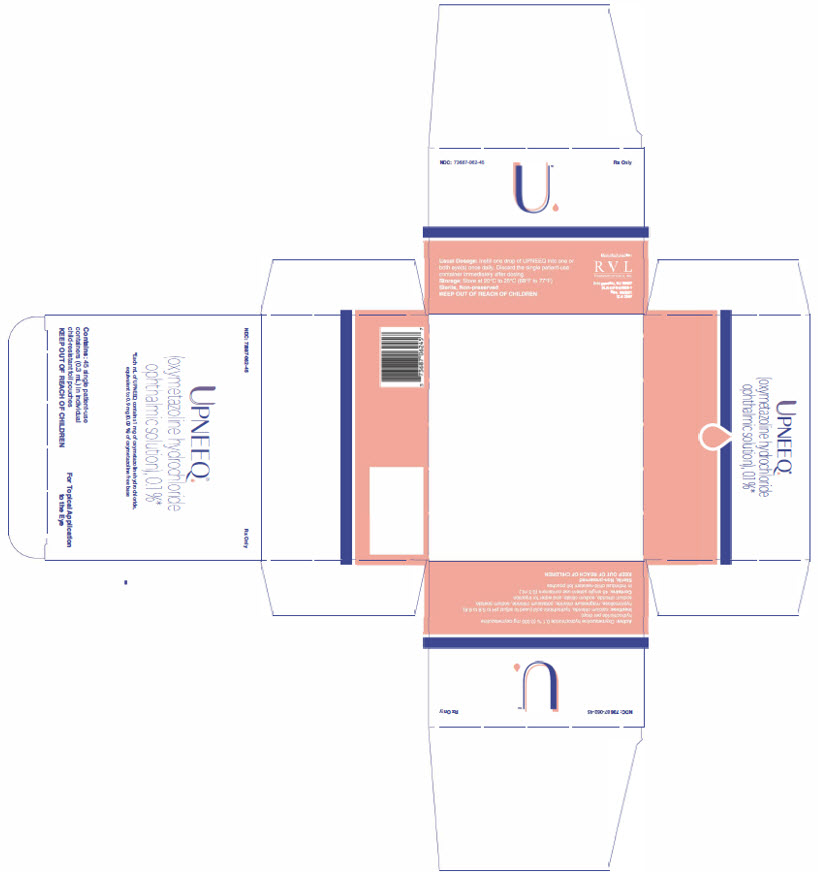
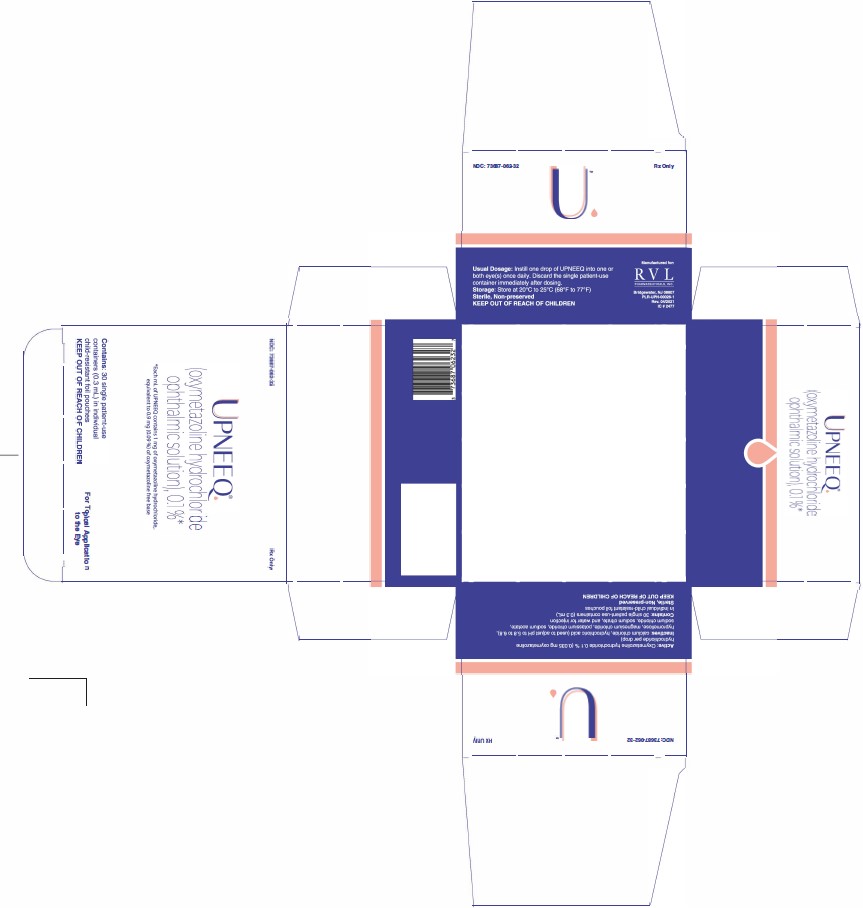
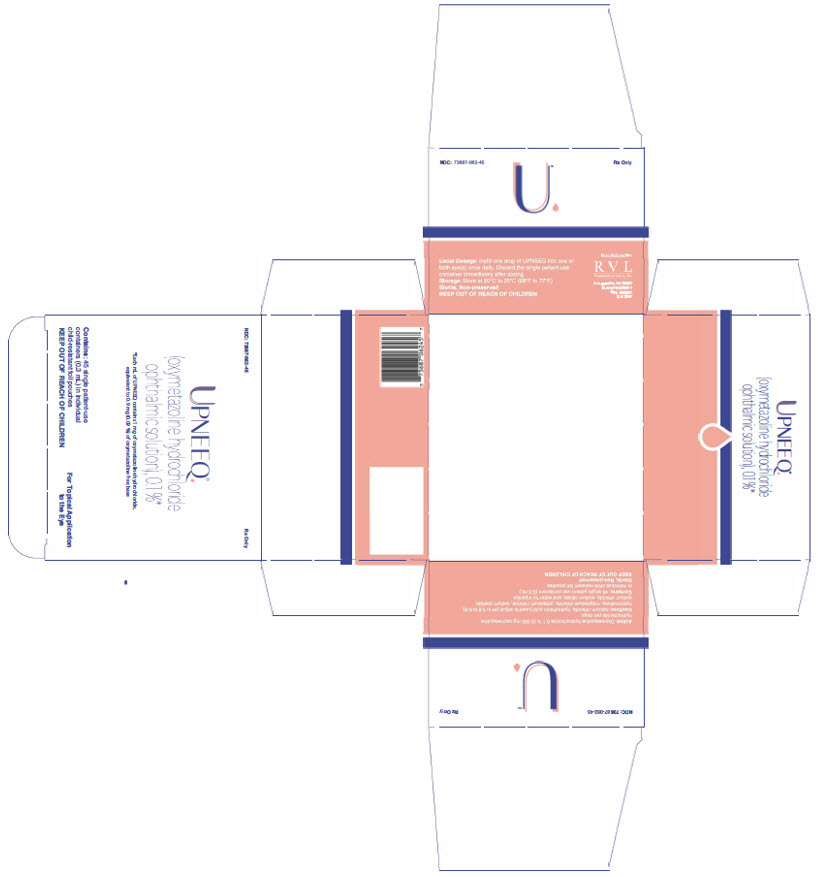
UPNEEQ (oxymetazoline hydrochloride ophthalmic solution), 0.1% is an aseptically prepared, sterile, non-preserved, clear, colorless to slightly yellow ophthalmic solution; 0.3 mL fill in a clear, low-density polyethylene, single patient-use container in a child-resistant foil pouch.
NDC 73687-062-32
Carton of 30 single patient-use containers in individual child-resistant foil pouches.
NDC 73687-062-45 Carton of 45 single patient-use containers in individual child-resistant foil pouches.
Storage: Store at 20°C to 25°C (68°F to 77°F). Protect from excessive heat. Keep out of reach of children.
Store single patient-use containers in the child-resistant foil pouches. Opened containers should be discarded immediately after use. -
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Instructions for Use).
When to Seek Medical Care
- Ptosis may be associated with neurologic or orbital diseases such as stroke and/or cerebral aneurysm, Horner syndrome, myasthenia gravis, external ophthalmoplegia, orbital infection and orbital masses. Consideration should be given to these conditions in the presence of ptosis with decreased levator muscle function and/or other neurologic signs [see Warnings and Precautions (5.1)].
- Alpha-adrenergic agonists as a class may impact blood pressure. Advise patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens [see Warnings and Precautions (5.2)].
- Use with caution in patients with cerebral or coronary insufficiency or Sjögren’s syndrome and advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop [see Warnings and Precautions (5.3)].
- Advise patients to seek immediate medical care if signs and symptoms of acute narrow-angle glaucoma develop [see Warnings and Precautions (5.4)].
Administration Instructions
Use with Contact Lenses
Advise patients that contact lenses should be removed prior to administration of UPNEEQ and can be re-inserted 15 minutes following administration [see Dosage and Administration (2)].Use with Other Ophthalmic Drugs
If more than one topical ophthalmic drug is being used, the drugs should be administered at least 15 minutes between applications.
Administration
Advise patients that the solution from one single patient-use container is to be used immediately after opening to dose one or both eye(s). The single patient-use container, including any remaining contents, should be discarded immediately after administration [see Dosage and Administration (2)].
Storage and Handling Instructions
Handling the Single Patient-Use Container
Advise patients not to touch the tip of the single patient-use container to their eye or to any surface, in order to avoid eye injury or contamination of the solution.Storage Information
Instruct patients to keep the single patient-use containers in the child-resistant foil pouches until ready to use. Keep out of reach of children.To report SUSPECTED ADVERSE REACTIONS, contact RVL Pharmaceuticals, Inc. at 1-877-482-3788 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
© 2023 RVL Pharmaceuticals, Inc. All rights reserved.
Bridgewater, New Jersey 08807
Patents 8357714, 9867808, 10799481, 10814001, 10898573, 10912765, 10940138, 11103482, 11311515, 11324722, and 11541036
Made in the U.S.A.
Manufactured for:
RVL Pharmaceuticals, Inc.
PLR-UPN-00007-7
IC # 2284 -
INSTRUCTIONS FOR USE
UPNEEQ® (up-NEEK)
(oxymetazoline hydrochloride ophthalmic solution), 0.1%
for topical ophthalmic useRead this Instructions for Use before you start using UPNEEQ and each time you get a refill. There
may be new information. This leaflet does not take the place of talking to your healthcare provider
about your medical condition or your treatment.UPNEEQ single patient-use container after removal from its individual child-resistant foil pouch
Important Information You Need to Know Before Using UPNEEQ
- UPNEEQ is for use in the eye.
- UPNEEQ can be harmful if swallowed.
- UPNEEQ is supplied as single patient-use containers individually packaged in a child-resistant foil pouch. Do not remove a single patient-use container from the child-resistant foil pouch until you are ready to use UPNEEQ.
- Wash your hands before each use to make sure you do not infect your eyes while using UPNEEQ.
- If you are using UPNEEQ with other eye (ophthalmic) medicines, you should wait at least 15 minutes between using UPNEEQ and the other medicines.
- If you wear contact lenses, remove them before using UPNEEQ. You should wait at least 15
minutes before placing them back into your eyes.
Using UPNEEQ
- Do not let the tip of the UPNEEQ single patient-use container touch your eye or any other surfaces to avoid contamination or injury to your eye.
- Use 1 drop of UPNEEQ in the affected eye 1 time each day.
- Each single patient-use container of UPNEEQ will give you enough medicine to treat both eyes 1 time each day if needed.
- There is some extra UPNEEQ in each single patient-use container in case you miss getting the drop into your eye.
Storing UPNEEQ
- Store UPNEEQ at room temperature between 68°F to 77°F (20°C to 25°C).
- Store UPNEEQ single patient-use containers in the child-resistant foil pouches they come in.
- Protect UPNEEQ from excessive heat.
- Keep UPNEEQ and all medicines out of the reach of children.
Disposing of UPNEEQ- After you have applied the daily dose of medicine, throw away (dispose of) the single patient-use container and any unused UPNEEQ.
- Do not save any unused UPNEEQ.
Follow Step 1 to Step 11 each time you use UPNEEQ:
Step 1. Remove 1 UPNEEQ child-resistant foil-pouch from the carton.
Step 2. Make sure the single patient-use container within the foil is moved completely away from the dotted line (see Figure A).
Step 3. Cut the foil along the dotted line using scissors (see Figure B).
Figure A
Figure B
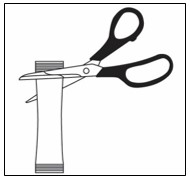
Step 4. Remove the single patient-use container from the foil pouch and then hold it by the long flat end (see Figure C).
Figure C
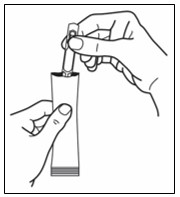
Step 5. Hold the single patient-use container upright and tap the top of the container gently to make sure that the medicine is in the bottom part of the container (see Figure D).
Figure D
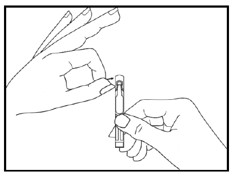
Step 6. Open the single patient-use container by twisting off the tab (see Figure E).
Do not let the tip of the container touch your eye or any other surfaces.
Figure E
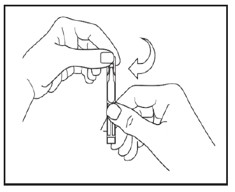
Step 7. Tilt your head backwards. If you are not able to tilt your head, lie down.
Step 8. Gently pull your lower eyelid downwards and look up (see Figure F).
Figure F
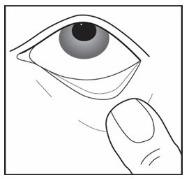
Step 9. Place the tip of the single patient-use container close to your eye, but be careful not to touch your eye with it (see Figure G).
Step 10. Gently squeeze the single patient-use container and let 1 drop of UPNEEQ fall into the space between your lower eyelid and your eye (see Figure G). If a drop misses your eye, try again.
Figure G
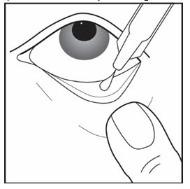
Step 11. Repeat Step 7 to Step 10 for your other eye if instructed to do so by your healthcare provider.
There is enough UPNEEQ in one container for both eyes if needed.
- After you have applied the daily dose of UPNEEQ, throw away the opened single patient-use container with any remaining medicine.
- Be sure to keep this medicine away from children.
- If you use contact lenses, wait at least 15 minutes before placing them back into your eyes.
Manufactured for:
RVL Pharmaceuticals, Inc.
Bridgewater, NJ 08807
1-877-482-3788
PLR-UPN-00007-7
This Instruction for Use has been approved by the U.S. Food and Drug Administration.
Approved: August 2020 - PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UPNEEQ
oxymetazoline hydrochloride ophthalmic solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73687-062 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM CHLORIDE (UNII: 660YQ98I10) SODIUM ACETATE (UNII: 4550K0SC9B) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73687-062-32 30 in 1 CARTON 08/21/2020 1 NDC:73687-062-70 .3 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC:73687-062-68 5 in 1 CARTON 10/19/2020 2 NDC:73687-062-80 .3 mL in 1 AMPULE; Type 0: Not a Combination Product 3 NDC:73687-062-34 30 in 1 CARTON 08/21/2020 3 NDC:73687-062-80 .3 mL in 1 AMPULE; Type 0: Not a Combination Product 4 NDC:73687-062-25 15 in 1 CARTON 08/21/2020 4 NDC:73687-062-80 .3 mL in 1 AMPULE; Type 0: Not a Combination Product 5 NDC:73687-062-45 45 in 1 CARTON 09/01/2020 5 NDC:73687-062-70 .3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212520 08/21/2020 Labeler - RVL Pharmaceuticals, Inc. (081365086)