Label: KILLFIRE MOUTH FRESHENER- cetylpyridinium chloride spray
- NDC Code(s): 24765-134-01
- Packager: Pharmacal-International. Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 17, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
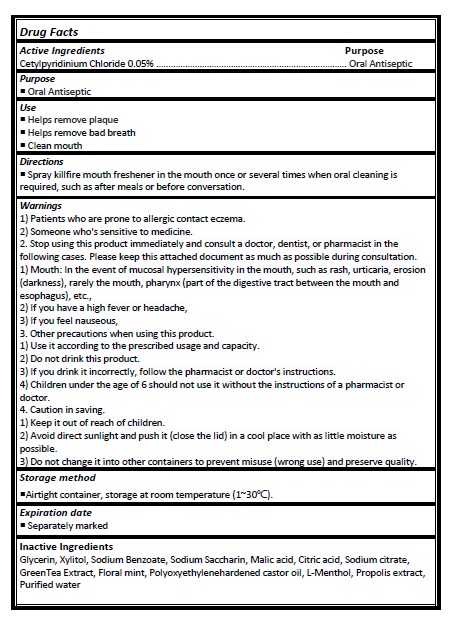
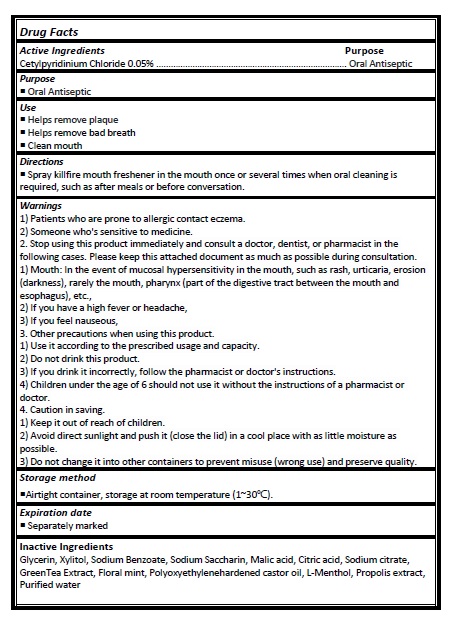
Active ingredient
Cetylpyridinium Chloride 0.05%
Directions
- Spray killfire mouth freshener in the mouth once or several times when oral cleaning is required, such as after meals or before conversation.
Warnings
1) Patients who are prone to allergic contact eczema.
2) Someone who's sensitive to medicine.
2. Stop using this product immediately and consult a doctor, dentist, or pharmacist in the following cases. Please keep this attached document as much as possible during consultation. 1) Mouth: In the event of mucosal hypersensitivity in the mouth, such as rash, urticaria, erosion (darkness), rarely the mouth, pharynx (part of the digestive tract between the mouth and esophagus), etc.,
2) If you have a high fever or headache,
3) If you feel nauseous,
3. Other precautions when using this product.
1) Use it according to the prescribed usage and capacity.
2) Do not drink this product.
3) If you drink it incorrectly, follow the pharmacist or doctor's instructions.
4) Children under the age of 6 should not use it without the instructions of a pharmacist or doctor.
4. Caution in saving.
1) Keep it out of reach of children.
2) Avoid direct sunlight and push it (close the lid) in a cool place with as little moisture as possible.
3) Do not change it into other containers to prevent misuse (wrong use) and preserve quality.
- Product label
-
INGREDIENTS AND APPEARANCE
KILLFIRE MOUTH FRESHENER
cetylpyridinium chloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24765-134 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) (CETYLPYRIDINIUM - UNII:CUB7JI0JV3) CETYLPYRIDINIUM CHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) XYLITOL (UNII: VCQ006KQ1E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MALIC ACID (UNII: 817L1N4CKP) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) GREEN TEA LEAF (UNII: W2ZU1RY8B0) RACEMENTHOL (UNII: YS08XHA860) WATER (UNII: 059QF0KO0R) PROPOLIS WAX (UNII: 6Y8XYV2NOF) POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24765-134-01 20 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 10/29/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/29/2021 Labeler - Pharmacal-International. Co., Ltd. (557805060) Registrant - Pharmacal-International. Co., Ltd. (557805060) Establishment Name Address ID/FEI Business Operations Ecoworld Co .,ltd 688735061 manufacture(24765-134)