BDR RE-VITAL PH PURE HARMONY CARE SOOTHING SKIN OPTIMIZER- panthenol liquid
Goldeneye Permanent System Gmbh Germany
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
GOLDENEYE - bdr Re-vital PH pure harmony care soothing skin optimizer
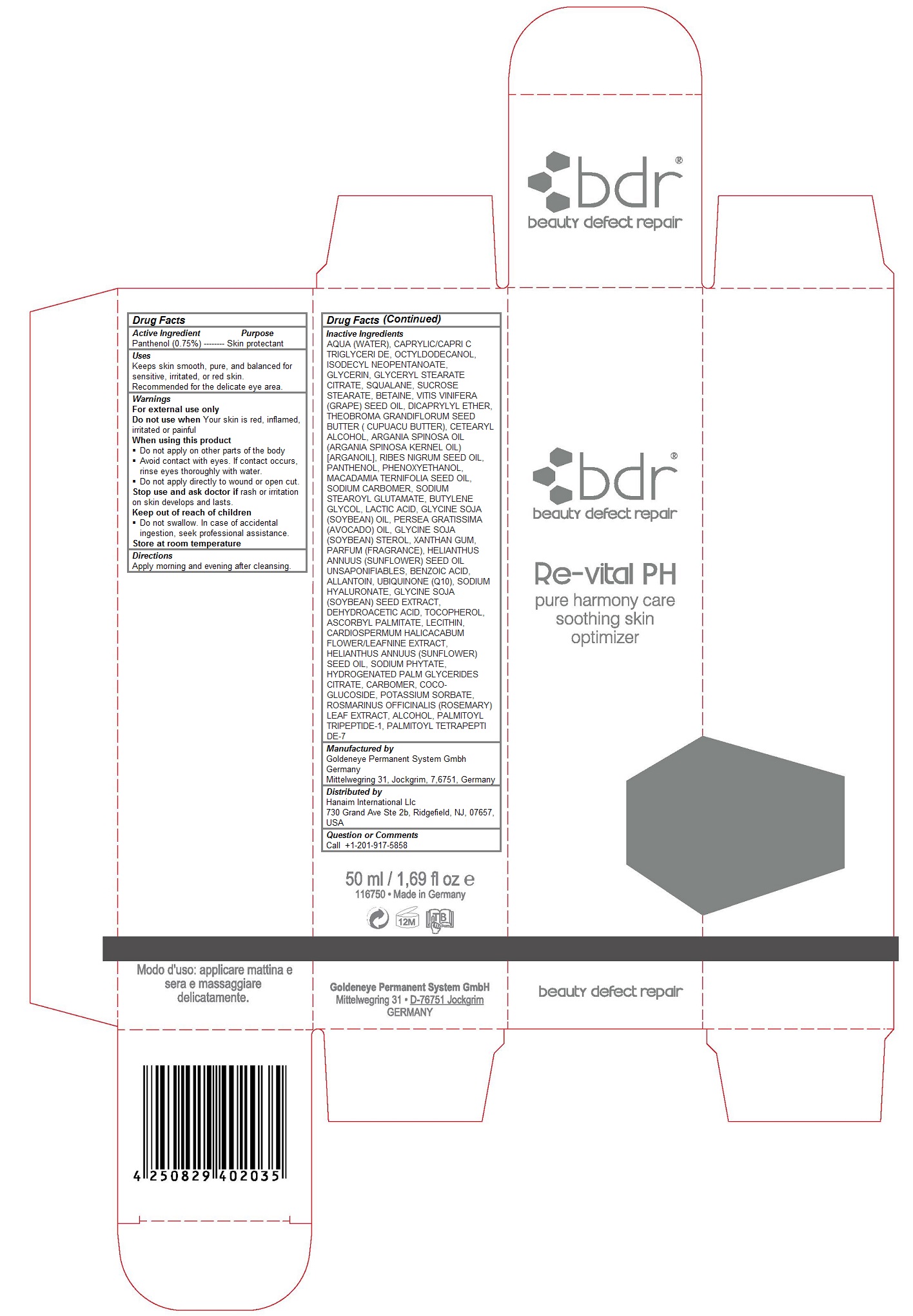
Keep out of reach of children
Do not swallow. In case of accidental ingestion, get medical help or contact a Poison Control Center right away.
Warnings
For external use only
Do not use when Your skin is red, inflamed, irritated or painful
When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on skin develops and lasts.
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
Store at room temperature
Uses
Keeps skin smooth, pure, and balanced for sensitive, irritated, or red skin. Recommended for the delicate eye area.
Inactive Ingredients
AQUA (WATER), CAPRYLIC/CAPRI C TRIGLYCERI DE, OCTYLDODECANOL, ISODECYL NEOPENTANOATE, GLYCERIN, GLYCERYL STEARATE CITRATE, SQUALANE, SUCROSE STEARATE, BETAINE, VITIS VINIFERA (GRAPE) SEED OIL, DICAPRYLYL ETHER, THEOBROMA GRANDIFLORUM SEED BUTTER ( CUPUACU BUTTER), CETEARYL ALCOHOL, ARGANIA SPINOSA OIL (ARGANIA SPINOSA KERNEL OIL) [ARGANOIL], RIBES NIGRUM SEED OIL, PANTHENOL, PHENOXYETHANOL, MACADAMIA TERNIFOLIA SEED OIL, SODIUM CARBOMER, SODIUM STEAROYL GLUTAMATE, BUTYLENE GLYCOL, LACTIC ACID, GLYCINE SOJA (SOYBEAN) OIL, PERSEA GRATISSIMA (AVOCADO) OIL, GLYCINE SOJA (SOYBEAN) STEROL, XANTHAN GUM, PARFUM (FRAGRANCE), HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL UNSAPONIFIABLES, BENZOIC ACID, ALLANTOIN, UBIQUINONE (Q10), SODIUM HYALURONATE, GLYCINE SOJA (SOYBEAN) SEED EXTRACT, DEHYDROACETIC ACID, TOCOPHEROL, ASCORBYL PALMITATE, LECITHIN, CARDIOSPERMUM HALICACABUM FLOWER/LEAFNINE EXTRACT, HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL, SODIUM PHYTATE, HYDROGENATED PALM GLYCERIDES CITRATE, CARBOMER, COCO-GLUCOSIDE, POTASSIUM SORBATE, ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT, ALCOHOL, PALMITOYL TRIPEPTIDE-1, PALMITOYL TETRAPEPTI DE-7
| BDR RE-VITAL PH PURE HARMONY CARE SOOTHING SKIN OPTIMIZER
panthenol liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Goldeneye Permanent System Gmbh Germany (329178144) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Goldeneye Permanent System Gmbh Germany | 329178144 | manufacture(71056-108) | |