CLINICALM- hydrocortisone cream
CP Skin Health Group, Inc.
----------
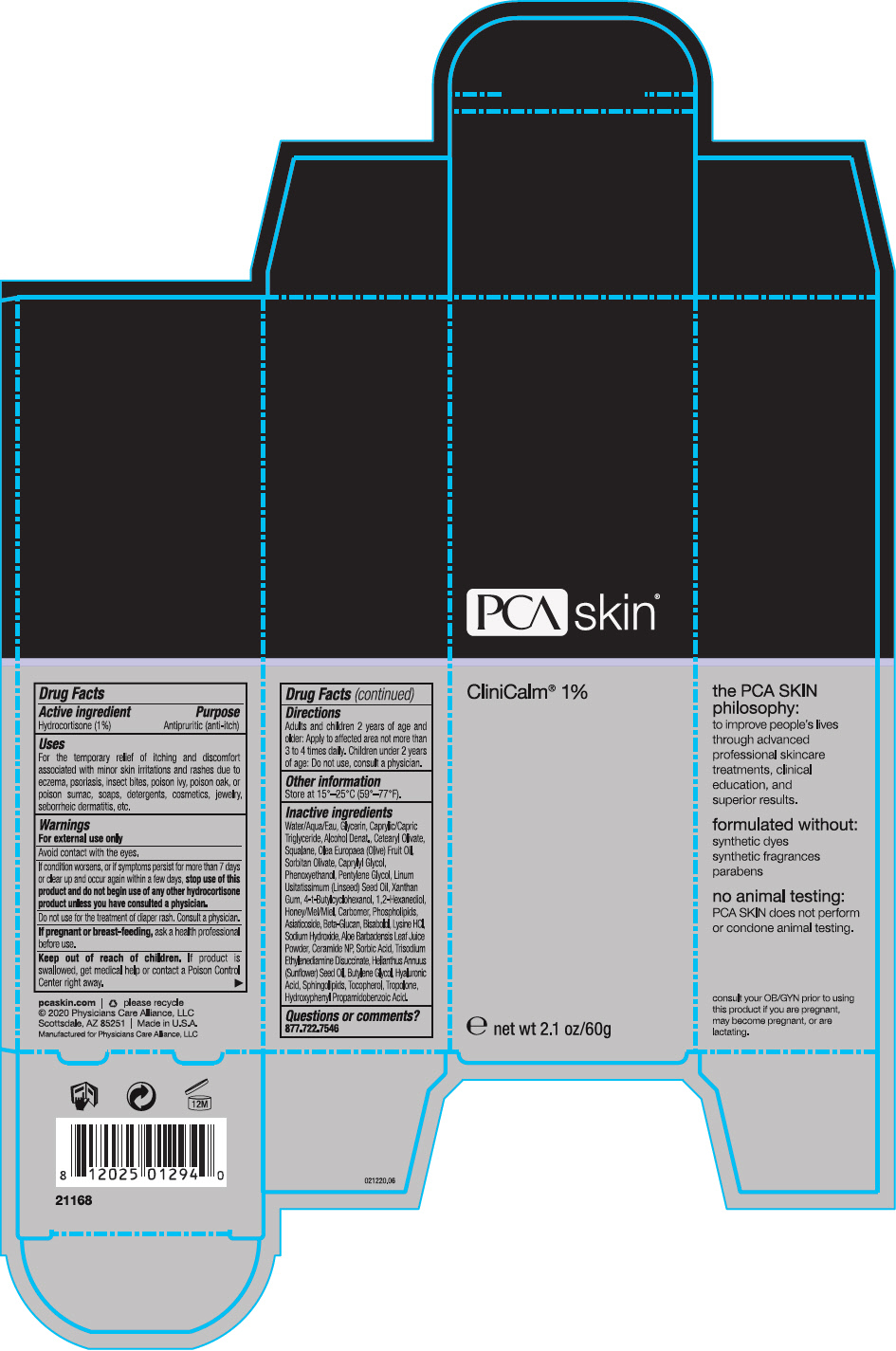
CliniCalm® 1%
Uses
For the temporary relief of itching and discomfort associated with minor skin irritations and rashes due to eczema, psoriasis, insect bites, poison ivy, poison oak, or poison sumac, soaps, detergents, cosmetics, jewelry, seborrheic dermatitis, etc.
Warnings
For external use only
Avoid contact with the eyes.
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, stop use of this product and do not begin use of any other hydrocortisone product unless you have consulted a physician.
Directions
Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily. Children under 2 years of age: Do not use, consult a physician.
Inactive ingredients
Water/Aqua/Eau, Glycerin, Caprylic/Capric Triglyceride, Alcohol Denat., Cetearyl Olivate, Squalane, Olea Europaea (Olive) Fruit Oil, Sorbitan Olivate, Caprylyl Glycol, Phenoxyethanol, Pentylene Glycol, Linum Usitatissimum (Linseed) Seed Oil, Xanthan Gum, 4-t-Butylcyclohexanol, 1,2-Hexanediol, Honey/Mel/Miel, Carbomer, Phospholipids, Asiaticoside, Beta-Glucan, Bisabolol, Lysine HCl, Sodium Hydroxide, Aloe Barbadensis Leaf Juice Powder, Ceramide NP, Sorbic Acid, Trisodium Ethylenediamine Disuccinate, Helianthus Annuus (Sunflower) Seed Oil, Butylene Glycol, Hyaluronic Acid, Sphingolipids, Tocopherol, Tropolone, Hydroxyphenyl Propamidobenzoic Acid.
| CLINICALM
hydrocortisone cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CP Skin Health Group, Inc. (611921669) |