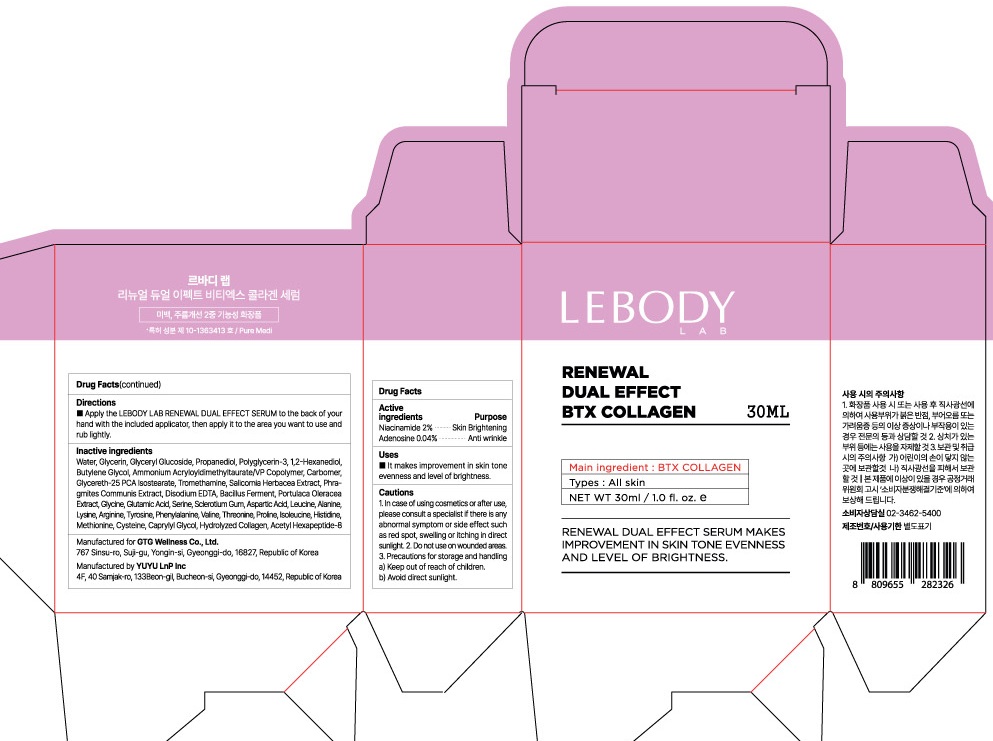
Label: LEBODY LAB RENEWAL DUAL EFFECT BTX COLLAGEN SERUM- niacinamide, adenosine liquid
- NDC Code(s): 71080-0012-1, 71080-0012-2
- Packager: GTG Wellness Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 24, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
-
INACTIVE INGREDIENTS
Water, Butylene Glycol, Dipropylene Glycol, Glycerin, 1,2-Hexanediol, Carbomer, Arginine, PEG-60 Hydrogenated Castor Oil, Ethylhexylglycerin, Thymus Vulgaris (Thyme) Extract, Origanum Vulgare Flower/Leaf/Stem Extract, Rosmarinus Officinalis (Rosemary) Extract, Lavandula Angustifolia (Lavender) Extract, Disodium EDTA, Gold, Xanthan Gum, Melaleuca Alternifolia (Tea Tree) Extract, Lavandula Angustifolia (Lavender) Oil, Cymbopogon Flexuosus Oil, Camellia Sinensis Leaf Extract, Sodium Hyaluronate
- PURPOSE
-
Cautions
1. In case of using cosmetics or after use, please consult a specialist if there is any abnormal symptom or side effect such as red spot, swelling or itching in direct sunlight.
2. Do not use on wounded areas.
3. Precautions for storage and handling
a) Keep out of reach of children.
b) Avoid direct sunlight. - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEBODY LAB RENEWAL DUAL EFFECT BTX COLLAGEN SERUM
niacinamide, adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71080-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 0.6 g in 30 mL Adenosine (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) Adenosine 0.012 g in 30 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71080-0012-2 1 in 1 CARTON 03/01/2019 1 NDC:71080-0012-1 30 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/01/2019 Labeler - GTG Wellness Co., Ltd. (689458057) Registrant - GTG Wellness Co., Ltd. (689458057) Establishment Name Address ID/FEI Business Operations YUYU LnP Inc 694752447 manufacture(71080-0012)