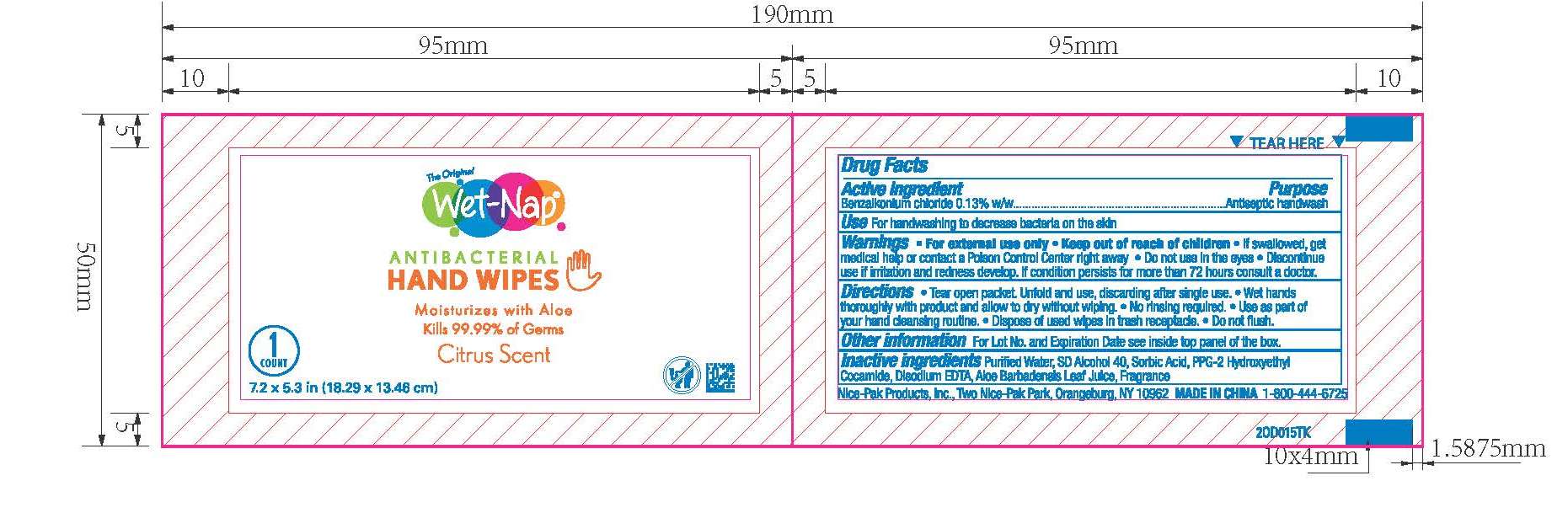
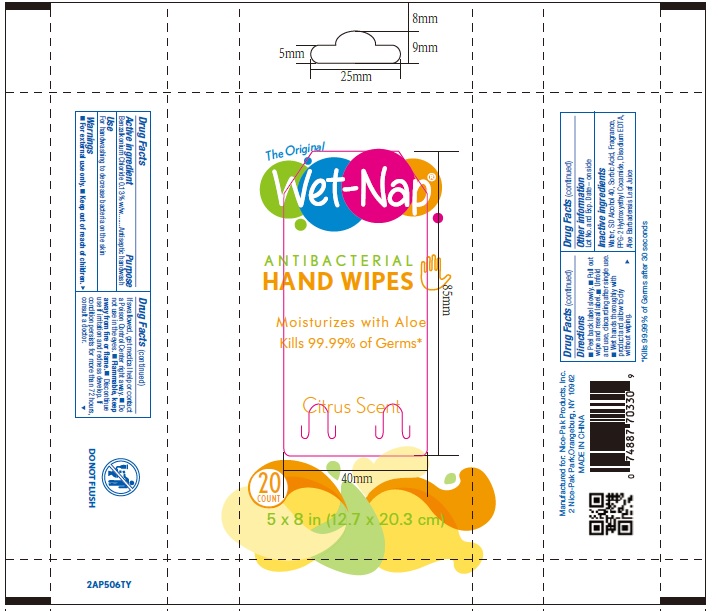
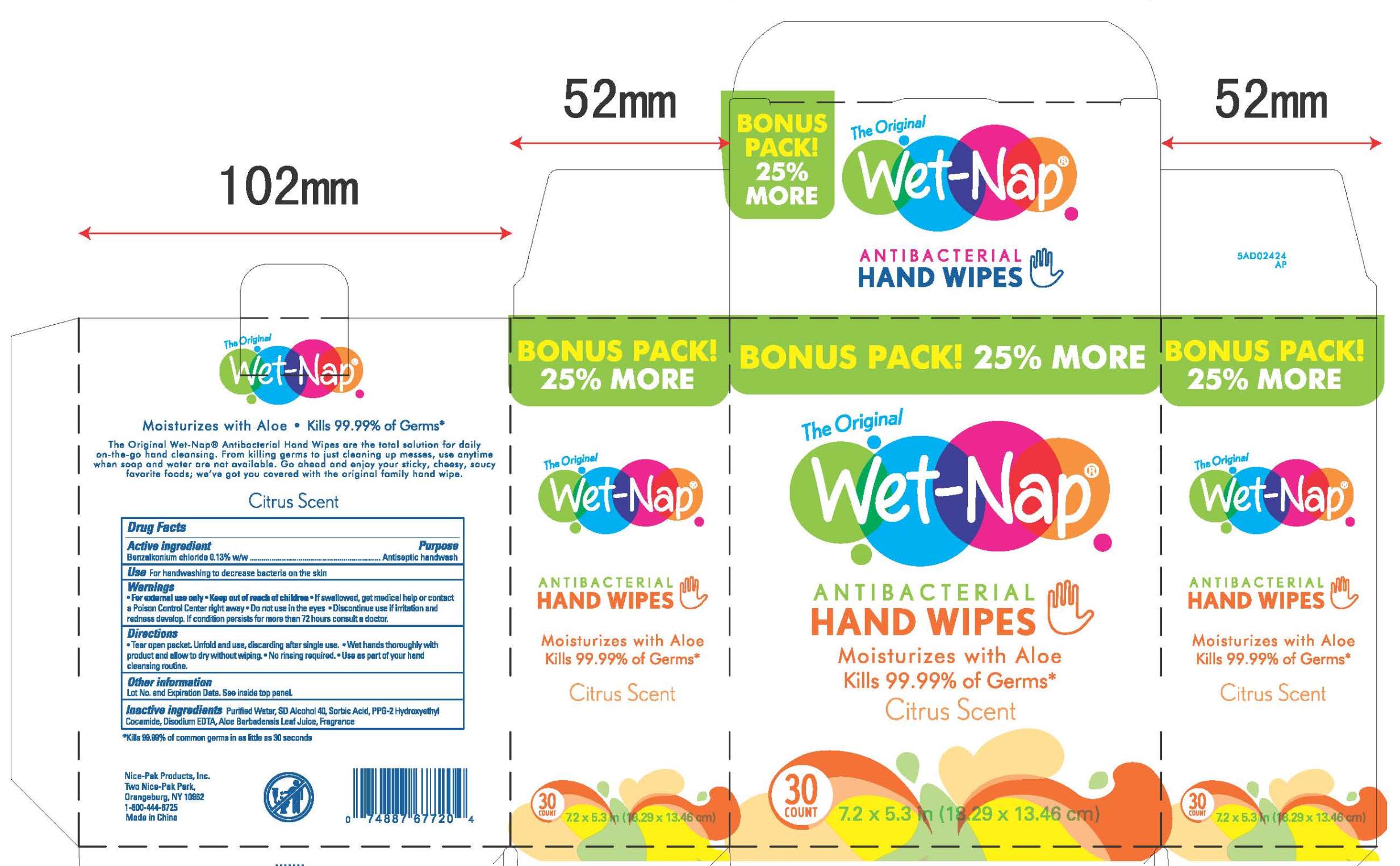
WET NAP ANTIBACTERIAL HAND WIPES- benzalkonium chloride cloth
Professional Disposables International Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Wet Naps Citrus NDC 10819-7012
Warnings
For external use only.
Do not use in the eyes.
Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Directions for container:
- To dispense, lift cover, remove seal, pull center sheet from roll, twist to a point, feed through dispenser hole in cover. Keep lid closed to prevent moisture loss.
- Use as part of your hand cleaning routine.
- Run product onto hands and allow to dry.
- Discard after single use.
Directions for packets or flowrap: see on container the directions to retreive the wipe.
| WET NAP
ANTIBACTERIAL HAND WIPES
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Professional Disposables International Inc. (800777117) |
| Registrant - Professional Disposables International Inc. (800777117) |
Revised: 10/2021
Document Id: cea74d62-c09a-f1a1-e053-2995a90a6fce
Set id: ce8d64e4-ace9-46a4-997a-d87d76fc46cd
Version: 7
Effective Time: 20211018
Professional Disposables International Inc.