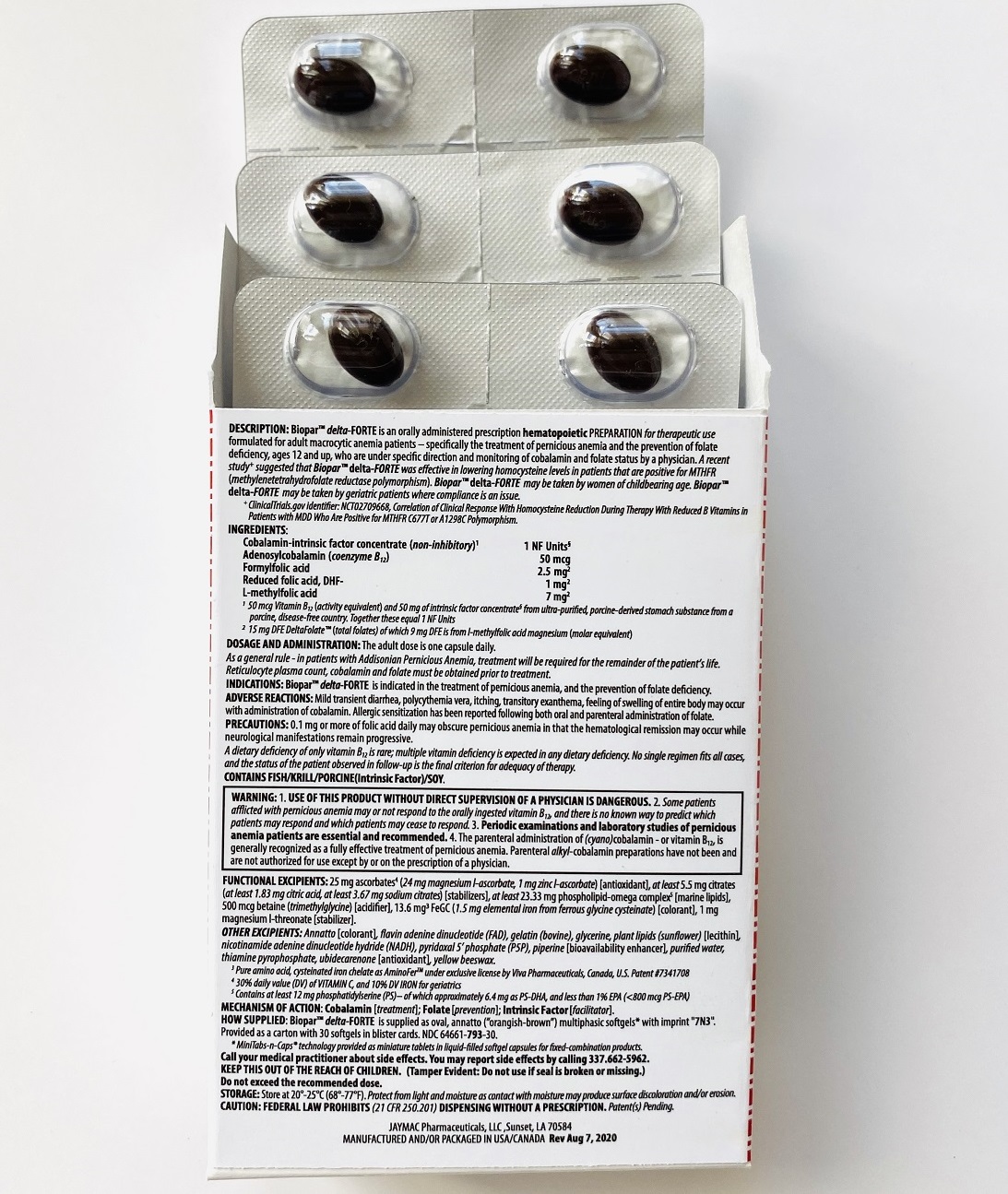
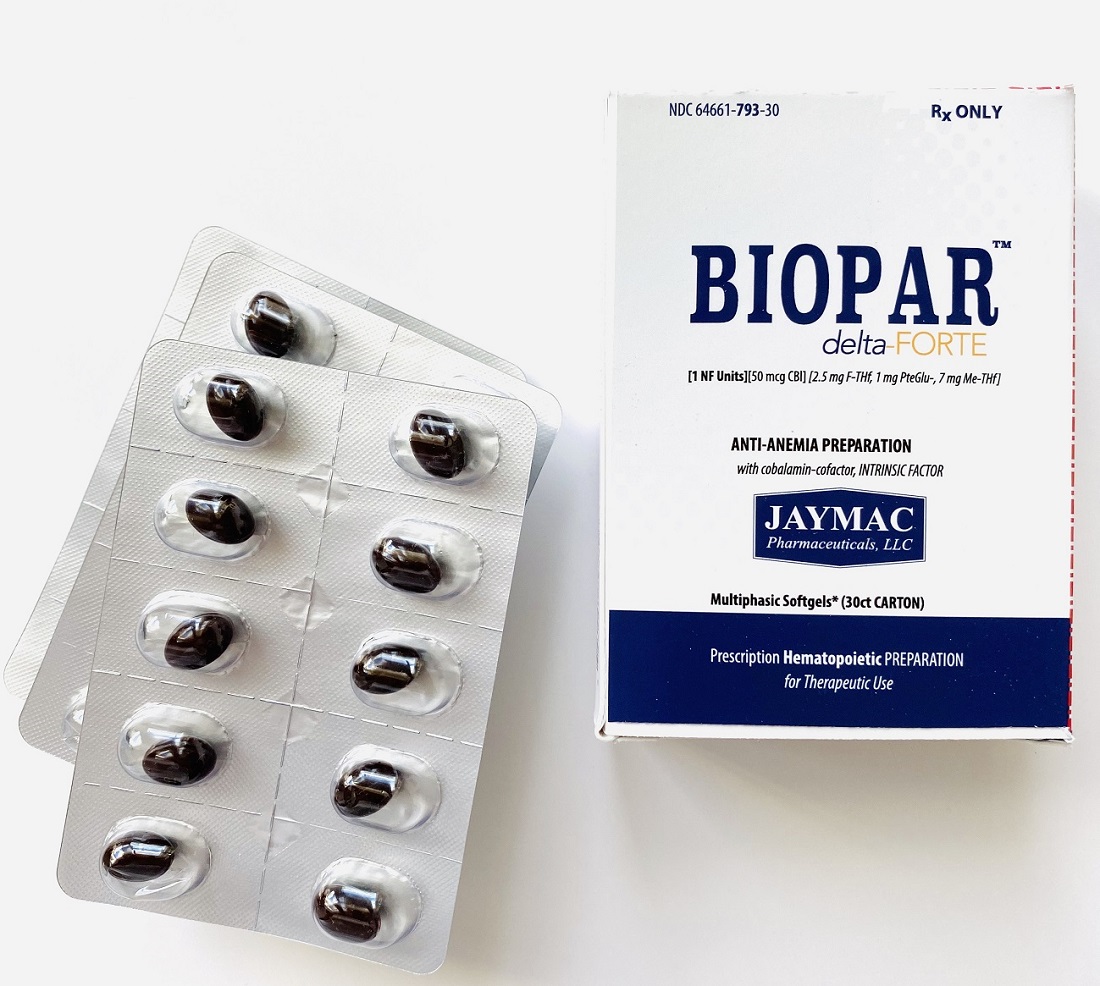
Label: BIOPAR DELTA-FORTE- 1 nf units, 50 mcg cbl, 2.5 mg f-thf, 1 mg pteglu-, 7 mg me-thf capsule
- NDC Code(s): 64661-793-30
- Packager: Jaymac Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 18, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
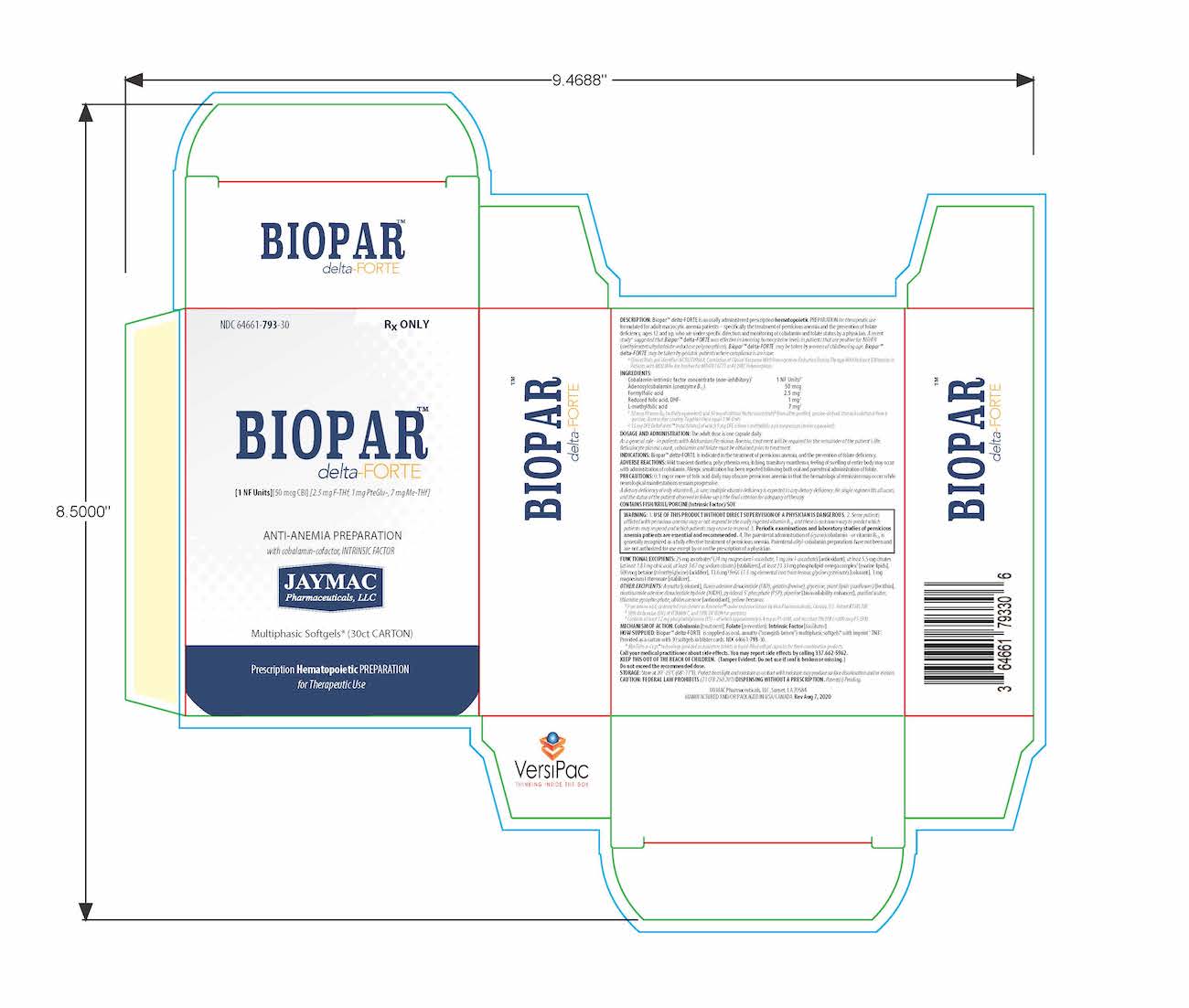
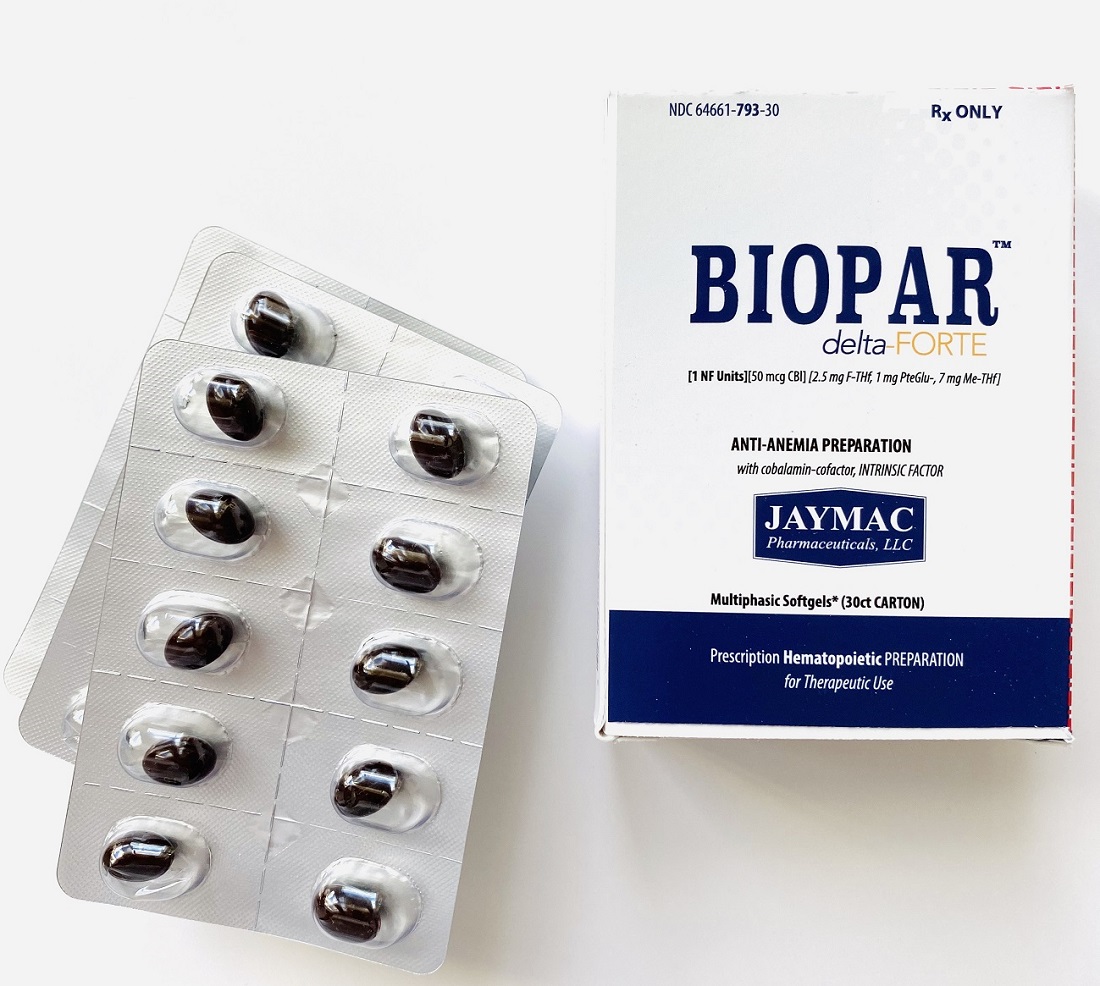
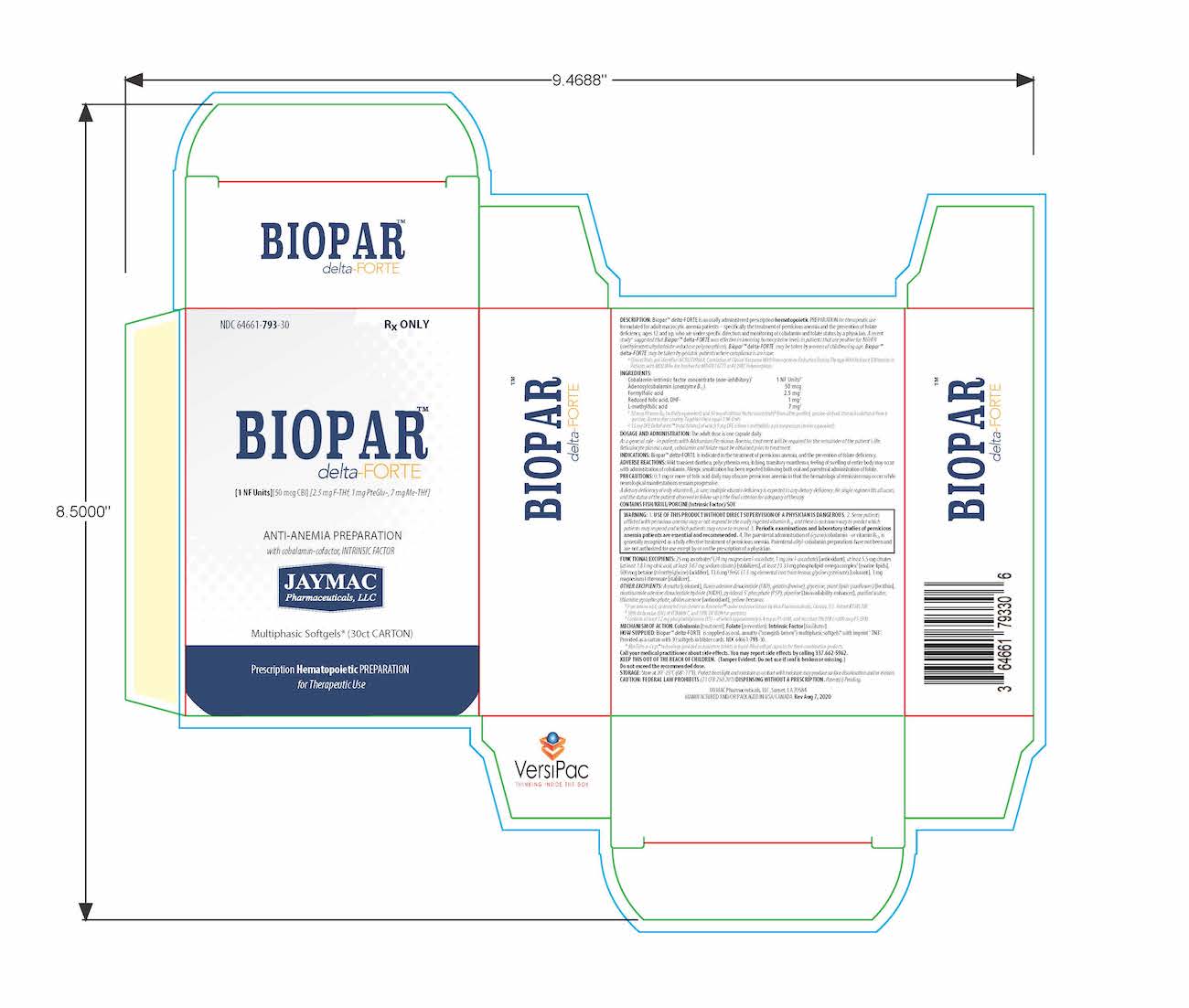
[1 NF Units] [50 mcg CBl] [2.5 mg F-THf] [1 mg PteGlu-] [7 mg Me-THf]
Prescription Hematopoietic Preparation For Therapeutic Use
Multiphasic Softgels (30ct carton)
NDC 64661-793-30
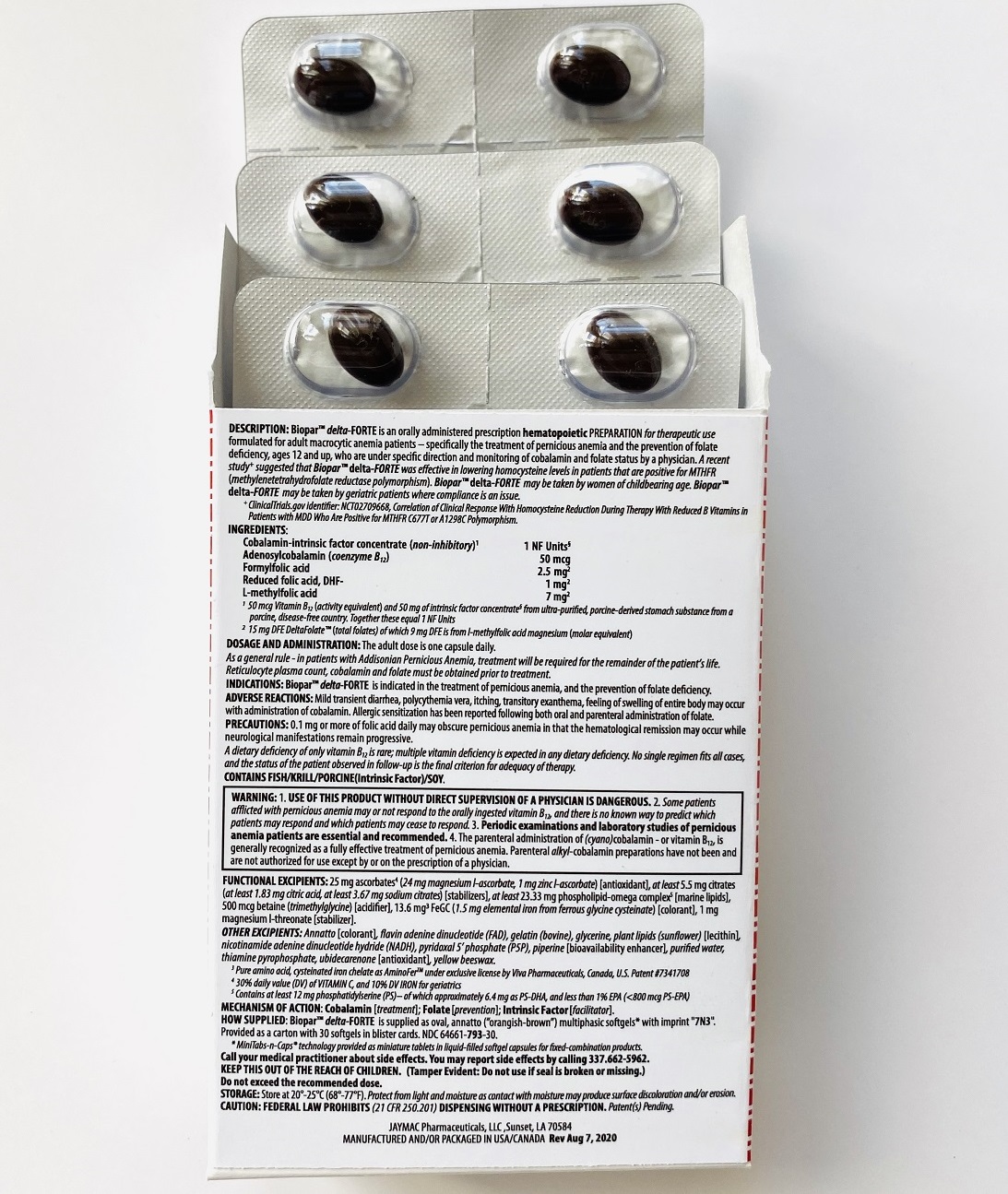
Rx ONLYBioparTMdelta-FORTE is an orally administered prescription hematopoietic preparation for therapeutic use formulated for adult macrocytic anemia patients – specifically including pernicious anemia patients, ages 12 and up, who are under specific direction and monitoring of cobalamin and folate status by a physician.
-
INGREDIENTS
Cobalamin-intrinsic factor concentrate (non-inhibitory)1 .............................................................................................. 1 NF Units§
Adenosylcobalamin (coenzyme B12) .............................................................................................................................. 50 mcg
Formyl folic acid, L-FTHf .............................................................................................................................................. 2.5 mg2
Oxidized folic acid, DHf+ ................................................................................................................................................. 1 mg2
Methyl folic acid, L-MeTHf ............................................................................................................................................... 7 mg2
1 50 mcg Vitamin B12 (activity equivalent) and 50 mg of intrinsic factor concentrate§ from ultra-purified, porcine-derived stomach substance from a porcine, disease-free country; together these equal 1 NF Units. Cobalamin has hematopoietic activity apparently identical to that of the anti-anemia factor in purified liver-stomach extract
2 Total folates is from l-methylfolate magnesium (molar equivalent) from amorphous, diastereoisomerically pure l-methylfolate (less than 1% d-isomer), DHF-dependent provitamin B9 (folic acid included) and the levo-isomer of folinic acid (label claim molar equivalent).
-
INACTIVES
ALSO CONTAINS:
25 mg ascorbates4a (24 mg magnesium l-ascorbate, 1 mg zinc l-ascorbate) [antioxidant], 13.6 mg chelates (Cys-Fe3 as cysteinated pure amino acid chelate subsisting of 1.5 mg elemental iron4a) [colorant], phospholipids (phosphatidylserine-docosahexaenoic acid complex5)
OTHER INGREDIENTS:Annatto [colorant], betaine (trimethylglycine) [acidifier], citrates (citric acid, sodium citrate) [stabilizers], flavin adenine dinucleotide (B2-vitamer)4b, gelatin (bovine), glycerine, nicotinamide adenine dinucleotide hydride (B3-vitamer)4b, phospholipids (sunflower lecithin) [emulsifiers], piperine [bioavailability enhancer], purified water, pyridoxal 5’ phosphate (B6-vitamer)4b, thiamine pyrophosphate (B1-vitamer)4b, ubidecarenone [antioxidant], yellow beeswax.
3 Pure amino acid, cysteinated iron chelate.
4a 30% daily value (DV) of VITAMIN C, and 10% DV IRON for geriatric patients.
4b Contains less than 2% (<25 mcg/each) of vitamins B1, B2, B3 and B6.
5 Contains at least 12 mg phosphatidylserine (PS) – of which approximately 6.4 mg as PS-DHA, and less than 1% EPA (<800 mcg PS-EPA)
- CONTAINS
- INDICATIONS & USAGE
-
MECHANISM OF ACTION
COBALAMIN [TREATMENT]; FOLATE [PREVENTION]; INTRINSIC FACTOR [B12-ADJUVENT] -
Cobalamin is essential for the synthesis of methionine from homocysteine - a reaction which also requires folate. In the absence of cobalamin - ie, cobalamin deficiency, tetrahydrofolate cannot be regenerated from l-methylfolic acid, and a functional folate deficiency occurs - ie, “methyl trap hypothesis”. Gastrointestinal absorption of cobalamin depends on the presence of sufficient intrinsic factor, and lack of intrinisic factor results in cobalamin deficiency.
-
DOSAGE & ADMINISTRATION
The adult dose is one capsule daily preferably on an empty stomach.
A deficiency of cobalamin may present first as folate deficiency - which is why folate supplementation may mask the symptoms that would normally result and also why advanced folate supplementation requires licensed medical supervision; because of this, reticulocyte plasma count, cobalamin and folate must be obtained prior to treatment. Requirements of cobalamin and/or folate in excess of normal (due to pregnancy, thyrotoxicosis, hemolytic anemia, hemorrhage, malignancy, hepatic and renal disease) can usually be met with oral supplementation.
As a general rule - in Pernicious Anemia patients, treatment will be required for the remainder of the patient’s life, and usually requires weekly or monthly injections at the doctor's office. Patients that are non-compliant with parenteral therapy (injections) may use this product as a substitute ONLY UNDER THE DIRECT SUPERVISION OF A LICENSED MEDICAL PRACTITIONER.
-
ADVERSE REACTIONS
Mild transient diarrhea, polycythemia vera, itching, transitory exanthema, feeling of swelling of entire body may occur with administration of cobalamin. Allergic sensitization has been reported following both oral and parenteral administration of folate.
Sensitivity to porcine intrinsic factor derived from liver substance has been reported in patients, and may occur at any time; BioparTMdelta-FORTE contains a non-inhibitory form of intrinsic factor that is not derived from liver substance but rather just the mucosa (stomach substance) - in which such senstitivity is less likely to occur, however caution is advised and continuous monitoring under licensed medical supervision is required.
-
BOXED WARNING
(What is this?)
WARNING
USE OF THIS PRODUCT WITHOUT DIRECT SUPERVISION OF A PHYSICIAN IS DANGEROUS;
Some patients afflicted with pernicious anemia may or not respond to the orally ingested vitamin B12, and there is no known way to predict which patients may respond and which patients may cease to respond;
Periodic examinations and laboratory studies of pernicious anemia patients are essential and recommended; and -
The parenteral administration of (cyano)cobalamin - or vitamin B12, is generally recognized as a fully effective treatment of pernicious anemia. Parenteral alkyl-cobalamin preparations have not been and are not authorized for use except by or on the prescription of a physician. -
PRECAUTIONS
GENERAL:
0.1 mg or more of folic acid daily may obscure pernicious anemia in that the hematological remission may occur while neurological manifestations remain progressive. The safe tolerable limit for folic acid (in preparations) is 1 mg [emphasis added];
Folic acid is not a substitute for vitamin B12 - although it may improve vitamin B12-deficient anemias. Exclusive use of folic acid in treating vitamin B12-deficient anemias could result in progressive and irreversible neurologic damage. Specifically, vitamin B12 deficiency allowed to progress over 3 months may produce permanent degenerative lesions of the spinal cord - as observed when folate therapy is used as the only hematopoietic agent;
Doses of vitamin B12 exceeding 10 mcg daily may produce hematologic response in patients with folate deficiency. Indiscriminate administration may mask the true diagnosis; and -
A dietary deficiency of only vitamin B12 is rare; multiple vitamin deficiency is expected in any dietary deficiency. No single regimen fits all cases, and the status of the patient observed in follow-up is the final criterion for adequacy of therapy.
DRUG INTERACTIONS:
Colchicine, para-aminosalicylic acid, and heavy alcohol intake for longer than 2 weeks may produce malabsorption of cobalamin.
Epileptic, antineoplastic and Parkinson's medications are cautioned in the concimant use with folate supplementation.
- HOW SUPPLIED
-
REGULATORY
BioparTM delta-FORTE is regulated as a drug to ensure it's use is under medical supervision, and because of this, advanced folate supplementation is possible [21 CFR 250.201]. Intrinsic Factor was first marketed as Extralin(R) in 1932, and Extralin-B(R) with B-vitamins in 1936, followed by the Becotin(R) product which contained all the equivalent vitamins as this product plus intrinsic factor. "Old" drugs, including virtamins, which were considered safe prior to 1938, were permitted to continue on the market without further review. However, FDA maintained the authority to review these old drugs if possible safety concerns became apparent. In 1951, the Durham-Humphrey Act was passed. This act formally differentiated between prescription and OTC drugs. - 44 FR 16131 (March 16, 1979).
-
STORAGE AND HANDLING
Call your medical practitioner about side effects. You may report side effects by calling (337) 662-5962.
KEEP THIS OUT OF THE REACH OF CHILDREN.
(Tamper Evident: Do not use if seal is broken or missing)
Do not exceed the recommended dose.
STORAGE: Store at 20 ̊-25 ̊ C (68 ̊-77 ̊ F)
CAUTION: FEDERAL LAW PROHIBITS DISPENSING WITHOUT A PRESCRIPTION
[Rx ONLY]
PATENTS: Patent(s) pending.
TRADEMARKS: BioparTM delta-FORTEis a registered mark of JayMac Pharmaceuticals. DeltaFolateTM is a use-trademark of Jaymac Pharmaceuticals.
JAYMAC Pharmaceuticals, LLC
Sunset, LA 70584
MANUFACTURED AND/OR PACKAGED IN USA/CANADA
Revision
Dec 28, 2021
- CARTON IMAGES
- PACKAGE INSERT
- PILL/CAPSULE IMAGE
- BLISTER CARD
-
INGREDIENTS AND APPEARANCE
BIOPAR DELTA-FORTE
1 nf units, 50 mcg cbl, 2.5 mg f-thf, 1 mg pteglu-, 7 mg me-thf capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64661-793 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INTRINSIC FACTOR (UNII: 70BT6OQT2Q) (INTRINSIC FACTOR - UNII:70BT6OQT2Q) INTRINSIC FACTOR 50 mg COBALAMIN (UNII: 8406EY2OQA) (COBALAMIN - UNII:8406EY2OQA) COBALAMIN 50 ug COBAMAMIDE (UNII: F0R1QK73KB) (COBAMAMIDE - UNII:F0R1QK73KB) COBAMAMIDE 50 ug levoLEUCOVORIN (UNII: 990S25980Y) (LEVOLEUCOVORIN - UNII:990S25980Y) levoLEUCOVORIN 2.5 mg DIHYDROFOLIC ACID (UNII: KXP0KNM559) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg LEVOMEFOLATE MAGNESIUM (UNII: 1VZZ62R081) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLIC ACID 7 mg Inactive Ingredients Ingredient Name Strength FERROUS CYSTEINE GLYCINATE (UNII: 8B4OP7RK5N) 13.6 mg 1,2-DOCOSAHEXANOYL-SN-GLYCERO-3-PHOSPHOSERINE CALCIUM (UNII: 6WJM73T46K) 6.4 mg 1,2-ICOSAPENTOYL-SN-GLYCERO-3-PHOSPHOSERINE CALCIUM (UNII: 9ABD9DRK7B) 800 ug PHOSPHATIDYL SERINE (UNII: 394XK0IH40) 12 mg PYRIDOXAL PHOSPHATE ANHYDROUS (UNII: F06SGE49M6) 25 ug FLAVIN ADENINE DINUCLEOTIDE (UNII: ZC44YTI8KK) 25 ug NADH (UNII: 4J24DQ0916) 25 ug COCARBOXYLASE (UNII: Q57971654Y) 25 ug MAGNESIUM ASCORBATE (UNII: 0N1G678593) 24 mg ZINC ASCORBATE (UNII: 9TI35313XW) 1 mg RIBOFLAVIN (UNII: TLM2976OFR) 5 mg MAGNESIUM L-THREONATE (UNII: 1Y26ZZ0OTM) 1 mg BETAINE (UNII: 3SCV180C9W) 500 ug CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 1.83 mg SODIUM CITRATE (UNII: 1Q73Q2JULR) 3.67 mg ANNATTO (UNII: 6PQP1V1B6O) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) OLIVE OIL (UNII: 6UYK2W1W1E) PIPERINE (UNII: U71XL721QK) WATER (UNII: 059QF0KO0R) UBIDECARENONE (UNII: EJ27X76M46) YELLOW WAX (UNII: 2ZA36H0S2V) Product Characteristics Color brown (ORANGISH-BROWN) Score no score Shape OVAL Size 16mm Flavor Imprint Code 7N3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64661-793-30 30 in 1 CARTON 06/01/2023 1 1 in 1 BLISTER PACK; Number of Units = 3; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/01/2023 Labeler - Jaymac Pharma (830767260) Registrant - Jaymac Pharma (830767260) Establishment Name Address ID/FEI Business Operations Viva Pharmaceuticals INC 253288898 manufacture(64661-793)