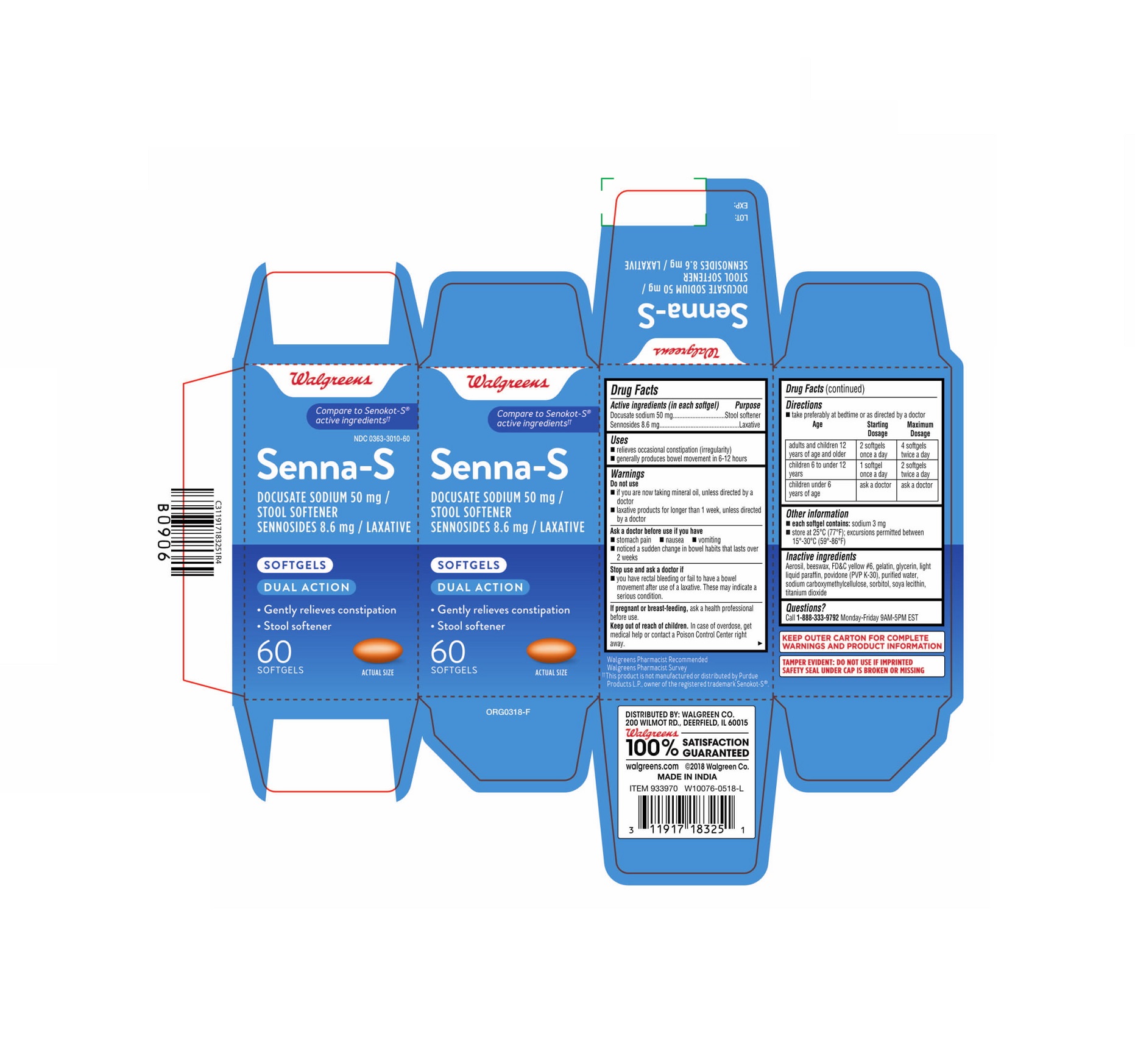
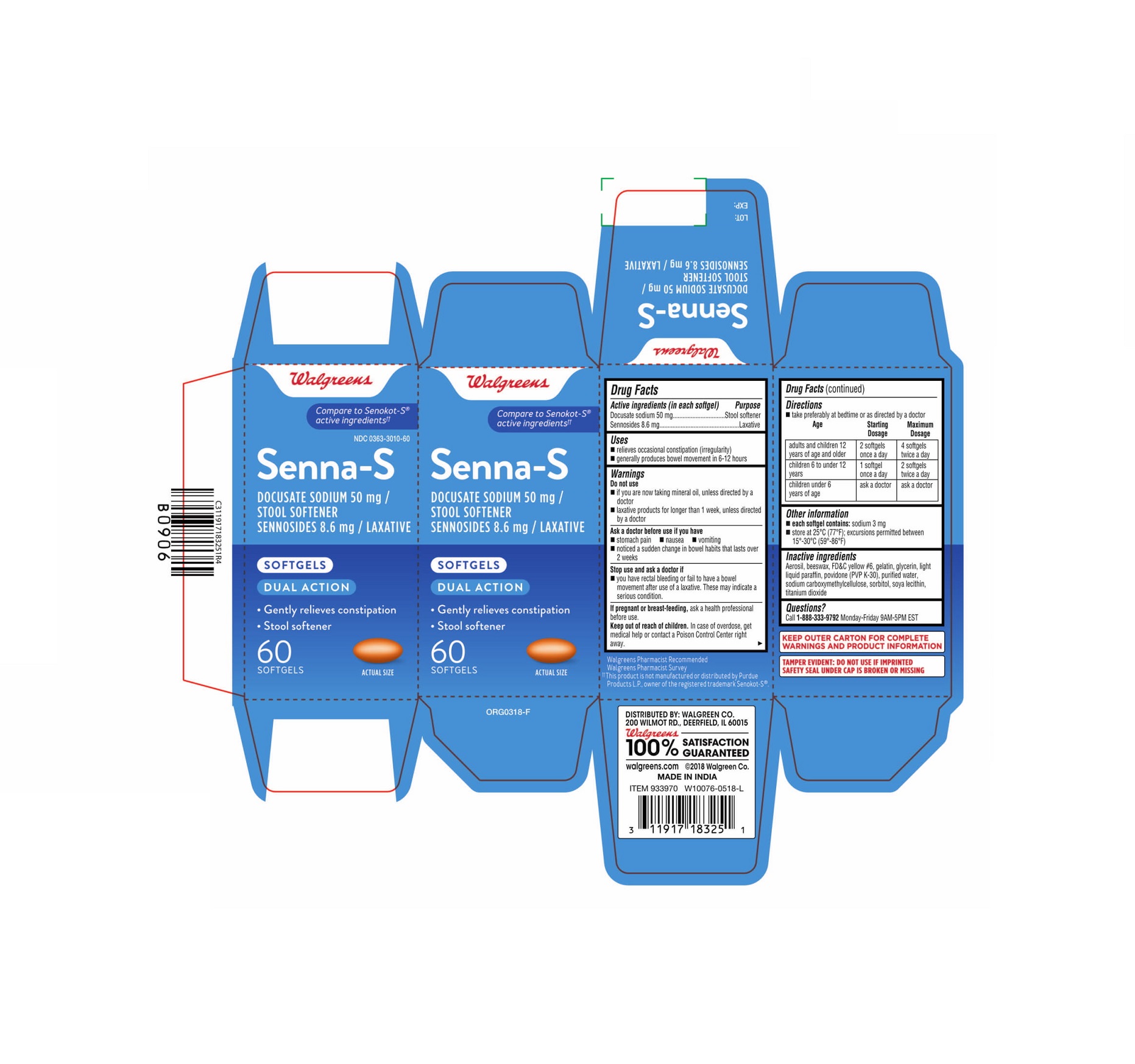
Label: SENNA-S- docusate sodium and sennosides capsule, gelatin coated
- NDC Code(s): 0363-3010-60
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week, unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 60 Softgels Bottle Carton
-
INGREDIENTS AND APPEARANCE
SENNA-S
docusate sodium and sennosides capsule, gelatin coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-3010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POVIDONE K30 (UNII: U725QWY32X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange (Opaque) Score no score Shape CAPSULE (SOFTGEL) Size 6mm Flavor Imprint Code S62 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-3010-60 1 in 1 CARTON 07/03/2018 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 03/01/2016 Labeler - Walgreens (008965063)