A2A HAND SANITIZER- alcohol gel
A2A INTEGRATED PHARMACEUTICALS, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
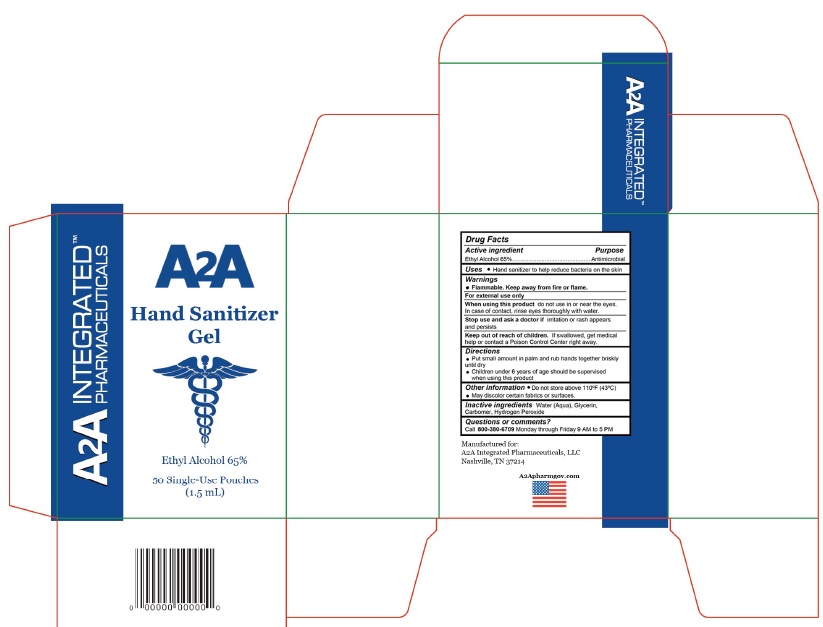
Flammable. Keep away from fire or flame.
For external use only
When using this product do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
S
top use and ask a doctor if irritation or rash appears and persists
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Put small amount in palm and rub hands together briskly until dry
Children under 6 years of age should be supervised when using this product
| A2A HAND SANITIZER
alcohol gel |
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| Labeler - A2A INTEGRATED PHARMACEUTICALS, LLC (117064671) |