MULTI VITAMIN, IRON AND FLUORIDE- multi vitamin, iron and fluoride solution/ drops
Mayne Pharma Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Multi Vitamin, Iron & Fluoride Supplemental Drops 0.25 mg Drops
DESCRIPTION
|
|||
| Each 1.0 mL supplies: | Percentage of U.S. Recommended Daily Allowance | ||
| Infants | Children Under 4 | ||
| Vitamin A | 1500 IU | 100 | 60 |
| Vitamin D | 400 IU | 100 | 100 |
| Vitamin E | 5 IU | 100 | 50 |
| Vitamin C | 35 IU | 100 | 88 |
| Thiamine | 0.5 mg | 100 | 71 |
| Riboflavin | 0.6 mg | 100 | 75 |
| Niacin | 8 mg | 100 | 89 |
| Vitamin B6 | 0.4 mg | 100 | 57 |
| Vitamin Iron> | 10 mg | 67 | 100 |
| Fluoride | 0.25 mg | * | * |
This product does not contain the essential vitamin folic acid.
See INDICATIONS AND USAGE section below for use by infants and young children 6 months to 3 years of age.
Active ingredient for caries prophylaxis: Each 1 mL contains 0.25 mg fluoride as sodium fluoride.
Other Ingredients: Ascorbic acid, caramel color, cholecalciferol, citric acid, D-alpha tocopheryl acid succinate, ferrous sulfate, flavor, glycerin, methylparaben, niacinamide, polysorbate 80, purified water, pyridoxine HCL, riboflavin-5-phosphate sodium, sodium benzoate, sodium hydroxide, sucralose, thiamine HCL, vitamin A palmitate.
CLINICAL PHARMACOLOGY
It is well established that fluoridation of the water supply (1 ppm fluoride) during the period of tooth development leads to a significant decrease in the incidence of dental caries.
Hydroxyapatite is the principal crystal for all calcified tissue in the human body. The fluoride ion reacts with the hydroxyapatite in the tooth as it is formed to produce the more caries-resistant crystal, fluorapatite. The reaction may be expressed by the equation:
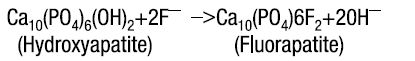
Three stages of fluoride deposition in tooth enamel can be distinguished:
- Small amounts (reflecting the low levels of fluoride in tissue fluids) are incorporated into the enamel crystals while they are being formed.
- After enamel has been laid down, fluoride deposition continues in the surface enamel. Diffusion of fluoride from the surface inward is apparently restricted.
- After eruption, the surface enamel acquires fluoride from water, food, supplementary fluoride and smaller amounts from saliva.
INDICATIONS AND USAGE
Supplementation of the diet with eight essential vitamins and iron, plus fluoride.
Supplementation of the diet with fluoride for caries prophylaxis.
The American Academy of Pediatrics recommends that children up to age 16, in areas where drinking water contains less than optimal levels of fluoride, receive daily fluoride supplementation. Multi Vitamin, Iron and Fluoride Supplemental Drops 0.25 provide fluoride in drop form for children ages 2-3 years where the drinking water contains less than 0.3 ppm of fluoride and for children over 3 years, in areas where the drinking water contains 0.3 through 0.7 ppm of fluoride. Each 1.0 mL provides sodium fluoride (0.25 mg fluoride) plus eight essential vitamins and iron.
The American Academy of Pediatrics and the American Dental Association currently recommend that infants and children under 2 years of age, in areas where drinking water contains less than 0.3 ppm of fluoride, and children 2-3, years of age, in areas where the drinking water contains 0.3 through 0.7 ppm of fluoride, receive 0.25 mg of supplemental fluoride daily which is provided in a full dose (1mL) of Multi Vitamin, Iron and Fluoride Supplemental Drops 0.25 mg.
Multi Vitamin, Iron and Fluoride Supplemental Drops 0.25 mg provide significant amounts of vitamins A, D, E, C thiamine, riboflavin, niacin, pyridoxine, and iron to supplement the diet, and to help assure that nutritional deficiencies of these vitamins will not develop. Thus, in a single easy-to-use preparation, children obtain eight essential vitamins and iron, plus fluoride.
PRECAUTIONS
The suggested dose should not be exceeded since dental fluorosis may result from continued ingestion of large amounts of fluoride. When prescribing vitamin fluoride products:
- Determine the fluoride content of the drinking water.
- Make sure the child is not receiving significant amounts of fluoride from other medications and swallowed toothpaste.
- Periodically check to make sure that the child does not develop significant dental fluorosis.
- Multi Vitamin, Iron and Fluoride Supplemental Drops 0.25 mg should be dispensed in the original plastic container, since contact with glass leads to instability and precipitation. (The amount of sodium fluoride in the 50-mL size is well below the maximum to be dispensed at one time according to recommendations of the American Dental Association.)
ADVERSE REACTIONS
Allergic rash and other idiosyncrasies have been rarely reported.
To report SUSPECTED ADVERSE REACTIONS, contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION
Infants and children under 2 years of age: 1.0 mL daily or as prescribed by physician. Multi Vitamin, Iron and Fluoride Supplemental Drops 0.25 mg may be dropped directly into mouth with the enclosed dropper or mixed with cereal, fruit juice or other food.
HOW SUPPLIED
Multi Vitamin, Iron and Fluoride Supplemental Drops 0.25 are available in 50 mL bottles with accompanying calibrated dropper, NDC 51862-163-50.
RECOMMENDED STORAGE:
Store at controlled room temperature, 20º-25ºC (68º-77ºF). [See USP Controlled Room Temperature]. After opening, store away from direct light. Close tightly after each use. REFRIGERATION IS NOT REQUIRED.
TAMPER EVIDENT: Do not use if printed bottle seal around bottle cap is broken or missing.
Manufactured for:
Libertas Pharma, Inc.
Lawrenceville, GA 30043
Iss. 03/11 163-50 743627
| MULTI VITAMIN, IRON AND FLUORIDE
multi vitamin, iron and fluoride solution/ drops |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Mayne Pharma Inc (867220261) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Medical Products Laboratories, Inc. | 002290302 | MANUFACTURE(51862-163) , ANALYSIS(51862-163) , LABEL(51862-163) , PACK(51862-163) | |
