Label: ASPIRIN suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 0574-7034-12, 0574-7036-12 - Packager: Padagis US LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 10, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Indications:
- Directions
- Caution:
- Keep this and all drugs out of the reach of children.
-
Warnings
CHILDREN AND TEENAGERS WHO HAVE OR ARE RECOVERING FROM CHICKEN POX, FLU SYMPTOMS, OR FLU SHOULD NOT USE THIS PRODUCT. IF NAUSEA, VOMITING, OR FEVER OCCUR, CONSULT A DOCTOR BECAUSE THESE SYMPTOMS COULD BE AN EARLY SIGN OF REYE SYNDROME, A RARE BUT SERIOUS ILLNESS.
As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product. IT IS ESPECIALLY IMPORTANT NOT TO USE ASPIRIN DURING THE LAST 3 MONTHS OF PREGNANCY UNLESS SPECIFICALLY DIRECTED TO DO SO BY A DOCTOR BECAUSE IT MAY CAUSE PROBLEMS IN THE UNBORN CHILD OR COMPLICATIONS DURING DELIVERY.
- ALCOHOL WARNING:
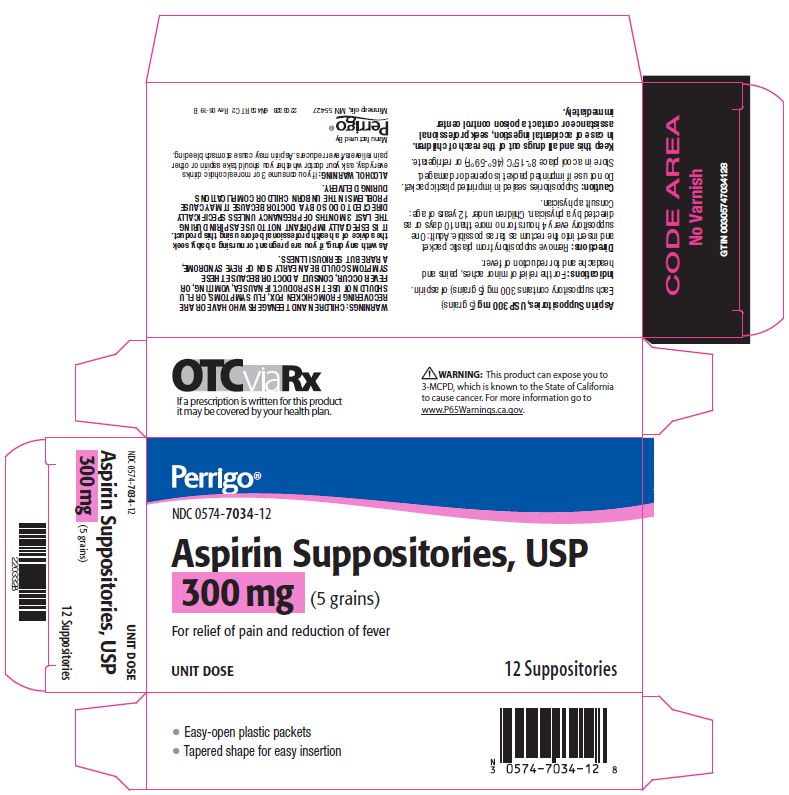
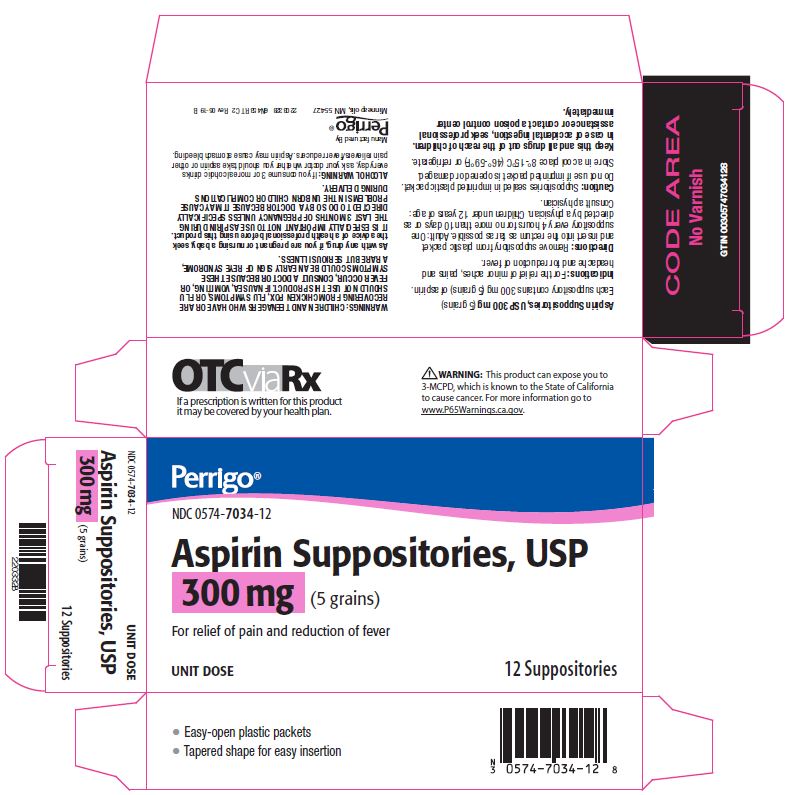
- Package/Label Principal Display Panel – 300 mg
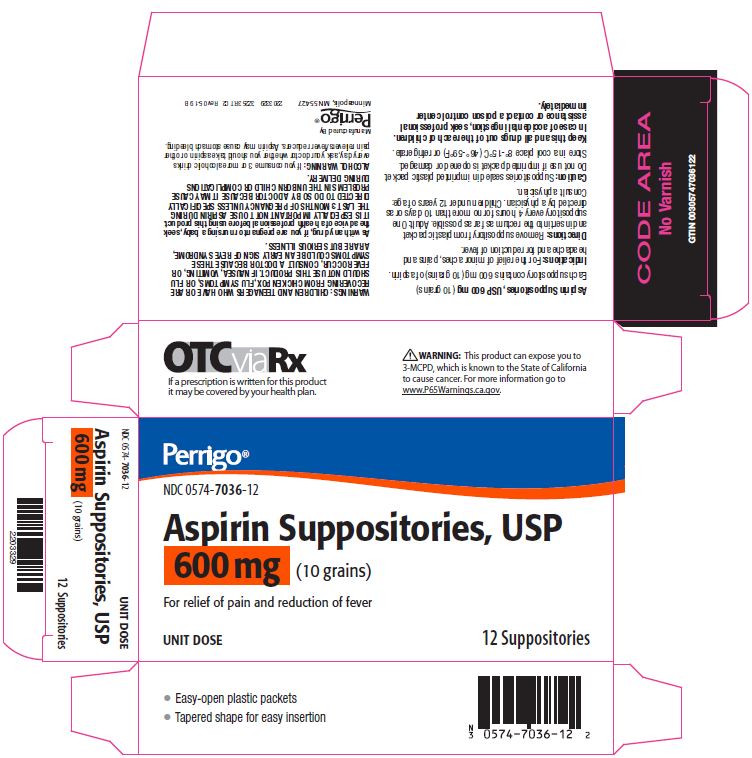
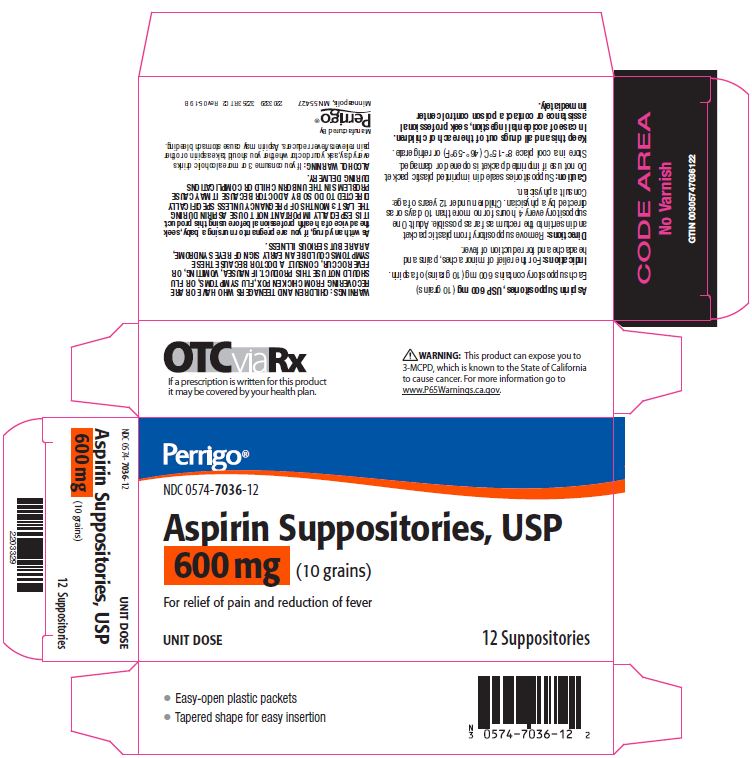
- Package/Label Principal Display Panel – 600 mg
-
INGREDIENTS AND APPEARANCE
ASPIRIN
aspirin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0574-7034 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 300 mg Inactive Ingredients Ingredient Name Strength HYDROGENATED PALM KERNEL OIL (UNII: FM8D1RE2VP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-7034-12 12 in 1 CARTON 09/01/1990 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 09/01/1990 ASPIRIN
aspirin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0574-7036 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 600 mg Inactive Ingredients Ingredient Name Strength HYDROGENATED PALM KERNEL OIL (UNII: FM8D1RE2VP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-7036-12 12 in 1 CARTON 09/01/1990 08/31/2021 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 09/01/1990 Labeler - Padagis US LLC (967694121)