Label: depodur- morphine sulfate injection, lipid complex
-
Contains inactivated NDC Code(s)
NDC Code(s): 24477-020-04, 24477-020-05 - Packager: Pacira Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
Drug Label Information
Updated January 10, 2008
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
-
DESCRIPTION
DepoDur (morphine sulfate extended-release liposome injection) is a sterile suspension of multivesicular liposomes using proprietary DepoFoam® formulation technology containing morphine sulfate, intended for epidural administration.
Chemically, morphine sulfate is 7, 8-didehydro-4, 5α-epoxy-17-methylmorphinan-3, 6α -diol sulfate (2:1) (salt) pentahydrate with a molecular weight of 758. Morphine sulfate pentahydrate has the following structural formula:
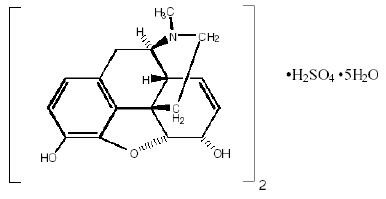
Morphine base has a pKa of 7.9, with an octanol/water partition coefficient of 1.42 at physiologic pH 7.4. At this pH, morphine’s tertiary amino group is mostly ionized, making the molecule water-soluble.
DepoDur is a sterile, non-pyrogenic, white to off-white, preservative-free suspension of multivesicular lipid-based particles containing Morphine Sulfate, USP. The median diameter of the liposome particles is in the range of 17 to 23 μm. The liposomes are suspended in a 0.9% sodium chloride solution. Each vial contains morphine sulfate (expressed as the pentahydrate) at a nominal concentration of 10 mg/mL. Inactive ingredients and their approximate concentrations are: 1,2-dioleoyl-sn-glycero-3‑phosphocholine (DOPC), 4.2 mg/mL; cholesterol, 3.3 mg/mL; 1,2-dipalmitoyl-sn-glycero-3‑phospho-rac-(1-glycerol) (DPPG), 0.9 mg/mL; tricaprylin, 0.3 mg/mL; and triolein, 0.1 mg/mL. The pH of DepoDur is in the range of 5.0 to 8.0.
After the administration of DepoDur into the epidural space, morphine sulfate is released from the multivesicular liposomes over a period of time.
Liposomal encapsulation or incorporation in a lipid complex can substantially affect a drug’s functional properties relative to those of the unencapsulated or nonlipid-associated drug. In addition, different liposomal or lipid-complexed products with a common active ingredient may vary from one another in the chemical composition and physical form of the lipid component. Such differences may affect functional properties of these drug products. DO NOT SUBSTITUTE.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Epidural administration of morphine sulfate results in analgesia without attendant loss of motor, sensory, or sympathetic function.
Morphine released from DepoDur is absorbed both neuraxially and systemically.
Morphine, a pure opiate agonist, is relatively selective for the μ-receptor, although it can interact with other opiate receptors at higher doses. In addition to analgesia, the widely diverse effects of morphine include respiratory depression, drowsiness, changes in mood, decreased gastrointestinal motility, nausea, vomiting, and alterations of the endocrine and autonomic nervous system.
Pharmacodynamics
The effects described below are common to all morphine-containing products.
Effects on the Central Nervous System (CNS): The principal therapeutic action of morphine is analgesia. Other therapeutic effects of morphine include anxiolysis, euphoria and feelings of relaxation. Although the precise mechanism of the analgesic action is unknown, specific CNS opiate receptors and endogenous compounds with morphine-like activity have been identified throughout the brain and spinal cord and are likely to play a role in the expression and perception of analgesic effects. As with all drugs in the opiate class, morphine can cause respiratory depression, in part by a direct effect on the brainstem respiratory centers. Morphine and related opiates depress the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia. Morphine may cause miosis, even in total darkness. Pinpoint pupils are a sign of opiate overdose; however, when asphyxia is present during opiate overdose, marked mydriasis occurs.
Effects on the Gastrointestinal Tract and on Other Smooth Muscle: Gastric, biliary and pancreatic secretions are decreased by morphine. Morphine causes a reduction in motility and is associated with an increase in tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone can be increased to the point of spasm, often resulting in constipation. Morphine can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi. Morphine may also cause spasm of the sphincter of the urinary bladder.
Effects on the Cardiovascular System: In therapeutic doses, morphine does not usually exert major effects on the cardiovascular system. Morphine, like other opiates, produces peripheral vasodilatation that may result in orthostatic hypotension and fainting. Release of histamine can occur, which may play a role in opiate-induced hypotension. Manifestations of histamine release and/or peripheral vasodilatation may include pruritus, flushing, red eyes and sweating.
Pharmacokinetics
Epidural administration of DepoDur results in both systemic absorption of morphine sulfate and absorption of morphine sulfate through the meninges into the intrathecal space. The relative absorption systemically versus intrathecally is unknown for DepoDur.
Absorption
Relative systemic bioavailability of DepoDur compared to epidurally administered morphine injection was determined in 19 patients (Table 1).
Table 1: Pharmacokinetics of DepoDur and Morphine Sulfate Injection (Mean ± SD) *Median (range)
DepoDur 5 mg
(n = 10)
Morphine Sulfate Injection
5 mg
(n = 9)
Parameter
Mean
SD
Mean
SD
Cmax (ng/mL)
7.1
3.4
23.8
12.8
tmax (hr)*
1.0
(0.3–4.0)
0.3
(0.3–2.0)
AUC (ng•hr/mL)
38.8
10.4
42.8
8.4
t1/2 (hr)
3.8
1.0
2.2
0.5
DepoDur systemic AUC was comparable to that of morphine sulfate injection (approximately 90%), however, systemic Cmax was 30 % of that of morphine sulfate injection.
Based on systemic AUC, DepoDur appears to exhibit dose proportionality over a dose range of 5 to 25 mg. In contrast, systemic Cmax did not exhibit dose-proportionality and tended to increase by an amount less than the proportional change in dose (Table 2).
Table 2: Morphine Plasma Pharmacokinetic Parameters (Mean, SD) Following Epidural Administration of DepoDur Parameter DepoDur
5 mg (n=14)DepoDur
10 mg (n=36)DepoDur
15 mg (n=71)DepoDur
20 mg (n=63)DepoDur
25 mg (n=32)DepoDur
30 mg (n=25)Cmax (ng/mL) 9.4 (5.7) 20.0 (9.5) 18.6 (10.4) 26.4 (18.6) 22.6 (15.4) 47.3 (28.9) AUC0–∞
(ng•hr/mL)41.0 (10.6) 124.9 (98.1) 131.6 (73.7) 185.9 (81.4) 207.3 (77.7) 341.5 (136.9) t1/2 (hr) 4.2 (2.1) 16.2 (19.7) 20.0 (20.6) 23.9 (25.4) 32.9 (24.2) 25.6 (14.6) Distribution
After morphine sulfate has been released from DepoDur and is absorbed systemically, morphine distribution is expected to be the same as for other morphine formulations.
Once absorbed, morphine is distributed to skeletal muscle, kidneys, liver, intestinal tract, lungs, spleen and brain. The volume of distribution of morphine is approximately 1 to 4 L/kg. Morphine is 20 to 35% reversibly bound to plasma proteins. Morphine also crosses the placental membranes and has been found in breast milk.
Metabolism
After morphine sulfate has been released from DepoDur and is absorbed systemically, morphine metabolism is expected to be the same as for other morphine formulations.
The major pathway of the detoxification of morphine is conjugation, either with D‑glucuronic acid in the liver to produce glucuronides or with sulfuric acid to give morphine-3-etheral sulfate. Although a small fraction (less than 5%) of morphine is demethylated, for all practical purposes, virtually all morphine is converted to glucuronide metabolites including morphine-3-glucuronide, M3G (about 50%) and morphine-6-glucuronide, M6G (about 5 to 15%). M3G has no significant analgesic activity. M6G has been shown to have opiate agonist and analgesic activity in humans.
Excretion
After morphine sulfate has been released from DepoDur and is absorbed systemically, morphine excretion is expected to be the same as for other morphine formulations. DepoDur is intended for single dose administration, therefore, accumulation of morphine or its metabolites is not expected even in patients with impaired hepatic or renal function.
Approximately 10% of morphine dose is excreted unchanged in the urine. Most of the dose is excreted in the urine as M3G and M6G. A small amount of the glucuronide metabolites is excreted in the bile and there is some minor enterohepatic cycling. Seven to 10% of administered morphine is excreted in the feces. The mean adult plasma clearance is about 20–30 mL/minute/kg. The effective terminal half-life of morphine after IV administration is reported to be approximately 2 hours. In some studies involving longer periods of plasma sampling, a longer terminal half-life of morphine of about 15 hours was reported.
Special Populations
Geriatric: Elderly patients (aged 65 years or older) may have increased sensitivity to DepoDur, as with other opiates. In elderly patients (over 65 years of age), the Cmax was similar to that of patients 65 years of age or younger, but the clearance in elderly patients was reduced by approximately 13%.
Hepatic Failure: Systemic morphine pharmacokinetics have been reported to be significantly altered in patients with cirrhosis. Clearance was found to decrease with a corresponding increase in half-life. The M3G and M6G to morphine plasma AUC ratios also decreased in these subjects, indicating diminished metabolic activity. DepoDur is intended for single-dose administration, therefore accumulation of morphine or its metabolites is not expected even in patients with impaired hepatic function.
Renal Insufficiency: Systemic morphine pharmacokinetics are altered in patients with renal failure. Clearance is decreased and the metabolites, M3G and M6G, may accumulate to much higher plasma levels in patients with renal failure as compared to patients with normal renal function. DepoDur is intended for single-dose administration, therefore accumulation of morphine or its metabolites is not expected even in patients with impaired renal function.
Drug-Drug Interactions: Other than the interactions with a lidocaine with epinephrine test dose (see below), no other pharmacokinetic drug-drug interactions have been examined in vivo. In-vitro studies suggest a similar interaction is anticipated with other amide local anesthetics. No in-vitro or in-vivo studies have been performed with ester local anesthetics. Known drug-drug interactions involving morphine are pharmacodynamic, not pharmacokinetic (see PRECAUTIONS, Drug Interactions).
Test Dose Interaction
Epidural administration of a 3-mL test dose (lidocaine 1.5% and epinephrine 1:200,000) may affect the release of morphine sulfate from DepoDur (see PRECAUTIONS, Drug Interactions). This issue has been examined in patients who received epidural administration of 15 mg of DepoDur at various time intervals after the test dose (Table 3). The test groups included a no-test-dose group and 3-, 10- and 15-minute delays between test dose and DepoDur administration. Additionally, saline flush after the test dose was assessed. The serum concentration of morphine was measured as a biomarker.
Table 3: Impact of Test Dose Administration on Peak Serum Concentration of DepoDur Minutes
Between Test
Dose and
DepoDur
AdministrationN
Mean tmax
hr (SD)
Mean Cmax
ng/mL (SD)
Median Cmax
ng/mL
Min–Max
ng/mL
No test dose
6
2.5 (2.1)
11.5 (7.4)
10.2
4.1–23.5
Flush + 3
8
0.2 (0.04)
30.2 (8.5)
31.4
15.8–40.1
Flush + 10
7
0.6 (0.7)
15.6 (9.3)
13.3
7.4–34.0
Flush + 15
8
0.5 (0.3)
11.4 (6.4)
11.4
2.0–20.0
No Flush + 3
8
0.4 (0.7)
25.6 (10.1)
22.6
15.2–45.4
Serum morphine Cmax was comparable to that of the no-test-dose group if DepoDur was administered 15 minutes after the test dose.
Anesthetics other than lidocaine with epinephrine have not been evaluated. -
Clinical Studies
The efficacy of DepoDur was demonstrated in four clinical trials comprised of 876 patients undergoing surgical procedures such as hip arthroplasty, prostatectomy, colon resection and cesarean section. In these clinical trials, efficacy was assessed for at least 48 hours and safety for up to 30 days after DepoDur administration.
Hip Arthroplasty
Two randomized, double-blind, placebo-controlled, parallel-group, dose-ranging studies evaluated the safety and efficacy of 10, 15, 20, 25, and 30 mg DepoDur in 314 patients undergoing hip arthroplasty. The mean age of patients was 59 years (range 18 to 88 years). Study medication was administered approximately 30 minutes before surgery. Post-operatively, patients self-administered intravenous fentanyl via patient-controlled analgesia (PCA) to maintain satisfactory analgesia.
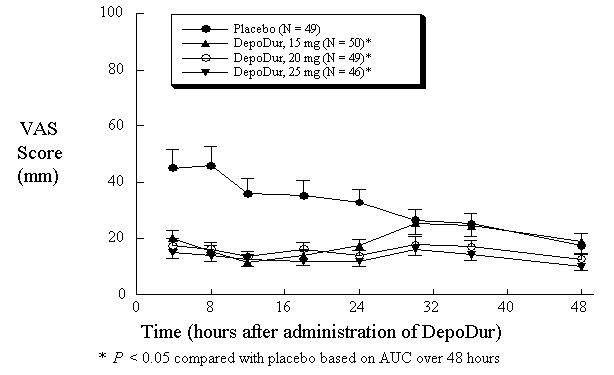
In one study (N=194), single epidural administration of 15, 20, and 25 mg DepoDur provided superior analgesic efficacy compared to placebo (epidural saline injection followed by IV fentanyl PCA), as measured by decreased fentanyl use (Figure 1) and Visual Analog Scores (VAS) (Figure 2). The second clinical study in hip arthroplasty revealed similar results.
Figure 1: Cumulative Fentanyl Usage Over 48 Hours (Mean, SE)
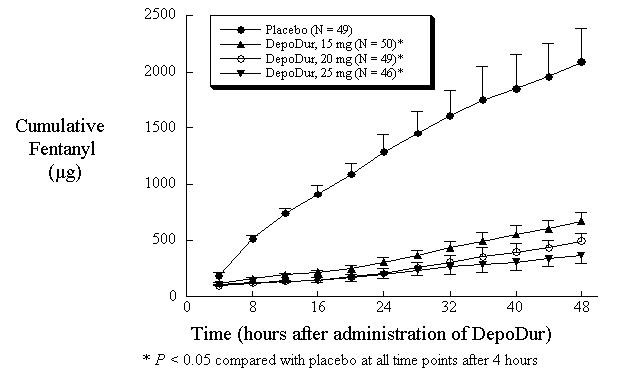
Figure 2:Pain Intensity Scores Over 48 hours (Mean, SE)
Lower Abdominal Surgery
A randomized, double-blind, parallel-group study evaluated the safety and efficacy of single epidural doses of 10, 15, 20, and 25 mg DepoDur compared to 5 mg DepoDur or 5 mg morphine sulfate in 487 patients undergoing lower abdominal surgery (i.e. surgery via an abdominal incision below umbilicus). Study medication was administered approximately 30 minutes prior to surgery. Post-operatively, patients self-administered intravenous fentanyl via patient-controlled analgesia (PCA) to maintain satisfactory analgesia. A dose response was observed, demonstrating a reduction in IV fentanyl use over the 48-hour period.
Cesarean Section
A randomized, double-blind, parallel-group study evaluated the safety and efficacy of single epidural doses of 5, 10, and 15 mg DepoDur compared to epidural morphine sulfate injection (5 mg) in 75 patients undergoing elective cesarean section under intrathecal anesthesia. Study medication was administered following delivery and clamping of the umbilical cord. At the investigator’s discretion, patients were permitted to receive acetaminophen with codeine or intravenous morphine sulfate as an intermittent bolus or via PCA pump, post-operatively. DepoDur doses of 10 and 15 mg resulted in reduced use of rescue medication and improved post-operative analgesia based on AUC analysis of VAS pain scores at rest (R) and with activity (A), compared to morphine sulfate on average over the 48-hour period following elective cesarean section (Table 4).
Table 4: Cesarean Section: Total Opiate Use, Pain VAS at Rest and with Activity *Median;†p< 0.05; § p< 0.001
Efficacy ParametersMorphine
Sulfate Injection
DepoDur5 mg 10 mg
15 mg
Opiate use (mg, morphine equivalents*) 0–48 h, median†
38.2
19.0
18.0
Opiate use (mg, morphine equivalents*) 24–48 h, median§
16.3
9.0
6.0
VAS-R AUC 0–48 h, mean ± SD†
1186 ± 939
454 ± 334 484 ± 425
VAS-A AUC 0–48 h, mean ± SD†
2086 ± 875
1235 ± 775
1036 ± 726
-
INDICATIONS AND USAGE
DepoDur is an extended-release liposome injection of morphine sulfate intended for single-dose administration by the epidural route, at the lumbar level, for the treatment of pain following major surgery. DepoDur is administered prior to surgery or after clamping the umbilical cord during cesarean section.
DepoDur is not intended for intrathecal, intravenous, or intramuscular administration. Administration of DepoDur into the thoracic epidural space or higher has not been evaluated and therefore is not recommended.
-
CONTRAINDICATIONS
DepoDur is contraindicated in patients with known hypersensitivity to morphine, morphine salts, or any components of the product. DepoDur, as with all opiates, is contraindicated in patients with respiratory depression, acute or severe bronchial asthma, and upper airway obstruction. Any contraindications for an epidural injection preclude the administration of DepoDur. DepoDur, as with all opiates, is contraindicated in any patient who has or is suspected of having paralytic ileus. DepoDur should not be used in patients with suspected or known head injury or increased intracranial pressure.
DepoDur is an opiate analgesic which causes vasodilatation that may exacerbate hypotension and hypoperfusion and, therefore, is contraindicated in circulatory shock.
-
WARNINGS
Due to the risk of severe adverse events when the epidural route of administration is employed, patients must be observed in a fully equipped and staffed environment for at least 48 hours after administration. The facility must be equipped to resuscitate patients with severe opiate overdosage, and the personnel must be familiar with the use and limitations of specific narcotic antagonists (naloxone, naltrexone) in such cases.
No clinical studies have evaluated the safety of administration of DepoDur into the intrathecal space. DepoDur is intended for administration by the epidural route only. However, cases of intrathecal administration of DepoDur have been reported during post-marketing experience. In all cases, signs of prolonged respiratory depression were observed requiring narcotic antagonist (naloxone) administration or ventilatory support.
Prolonged and serious respiratory depression or apnea has occurred when administration of epidural DepoDur was associated with subarachnoid puncture. In these cases, respiratory depression has occurred within 12 hours of DepoDur administration following apparent recovery from anesthesia. Respiratory depression resulting from DepoDur can be treated successfully with a naloxone bolus or, more commonly, a naloxone infusion; intubation and mechanical ventilation may be necessary in some cases.
Because intrathecal leakage from the epidural space may occur through a breached dural membrane, especially when the epidural drug is administered in a bolus, DepoDur should not be administered to a patient following a recent dural puncture without vigilant monitoring of respiratory function for a prolonged period (48 hours) with provision for emergency mechanical ventilation to minimize the risk of serious respiratory depression.
DepoDur should be administered by or under the direction of a physician experienced in the techniques associated with epidural drug administration and familiar with patient management following epidural opiate administration, including the management of respiratory depression.
Prior to drug administration, the physician should be familiar with patient conditions (such as infection at the injection site, bleeding diathesis, current and anticipated anticoagulant therapy, etc.) that call for special evaluation of the benefit versus risk potential.
Impaired Respiration
Respiratory depression is the chief hazard of all opiate preparations. Respiratory depression occurs more frequently in elderly or debilitated patients and in those suffering from conditions accompanied by hypoxia or hypercapnia in whom even moderate therapeutic doses may significantly decrease pulmonary ventilation.
Administration of opiates with or without coadministration of other sedative or hypnotic drugs can worsen airway obstruction in patients with obstructive sleep-apnea syndrome. Patients who are obese are at particular risk for this syndrome, which may be undiagnosed prior to administration.
Respiratory depression can occur with DepoDur. Four percent of the patients who received DepoDur required treatment with narcotic antagonists for respiratory depression. Ninety percent of events of respiratory depression started within the first 24 hours after dosing with DepoDur. However, the incidence of respiratory depression starting after 48 hours, potentially related to DepoDur, was 0.6% (5 of 900 patients). Patients at increased risk of respiratory depression such as those with impaired respiratory drive, sleep apnea, concomitant sedation or the elderly, may require monitoring for periods longer than 48 hours.
Because of the risk of respiratory depression, the facility must be equipped to resuscitate patients. Patients must be closely monitored in a fully equipped and staffed environment for a minimum of 48 hours.
If the surgical procedure is cancelled after the administration of DepoDur, the risk of respiratory depression may be increased, and patients should be monitored with a high level of vigilance.
Epidural local anesthetics should not be used before or after DepoDur, except in the form of a test dose. (See PRECAUTIONS – Drug Interactions) Do not mix or co-administer DepoDur with any other medications including local anesthetics. Once DepoDur has been administered, no other medication should be administered into the epidural space for at least 48 hours.
Misuse, Abuse and Diversion of Opiates
The active ingredient of DepoDur is morphine, a μ-opiate agonist. DepoDur is a Schedule II controlled substance. Such drugs are sought by drug abusers and people with addiction disorders. Diversion of Schedule II products is an act subject to criminal penalty. DepoDur can be abused in a manner similar to other opiate agonists, legal or illicit.
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Hypotensive Effect
DepoDur, like all other opiates, may cause severe hypotension in an individual whose ability to maintain blood pressure has already been compromised by a depleted blood volume or concurrent administration of drugs such as phenothiazines or general anesthetics (see also PRECAUTIONS – Drug Interactions). DepoDur may produce orthostatic hypotension and syncope in ambulatory patients.
In a patient who progresses to circulatory shock, DepoDur may make resuscitation more difficult due to vasodilatation.
Gastrointestinal Obstruction
DepoDur should not be administered to patients with gastrointestinal obstruction, especially paralytic ileus, because DepoDur diminishes propulsive peristaltic waves in the gastrointestinal tract and may prolong the obstruction.
Use with Other Central Nervous System Depressants
The central nervous depressant effects of morphine are potentiated by the presence of other CNS depressants such as alcohol, other opiates, sedatives, antihistaminics or psychotropic drugs. Use of neuroleptics or general anesthetics in conjunction with epidural morphine may increase the risk of respiratory depression.
-
PRECAUTIONS
General
Epidural delivery of opiates is accompanied by risk to the patients and requires a high level of skill to be successfully accomplished. The task of treating these patients must be undertaken by experienced clinical teams, well-versed in patient selection and emerging standards of care. It is critical to adjust the dose of DepoDur for each individual patient, taking into account the patient’s prior experience with opiate analgesics (see DOSAGE AND ADMINISTRATION).
Seizures may result from high doses of morphine. Patients with known seizure disorders should be carefully observed for evidence of morphine-induced seizure activity.
Use in Hepatic or Renal Disease
After morphine sulfate has been released from DepoDur and is absorbed systemically, its distribution, metabolism and excretion are expected to be the same as other morphine formulations. DepoDur is intended for single-dose administration; therefore accumulation of morphine or its metabolites is not expected even in patients with impaired hepatic or renal function.
Use in Biliary Surgery or Disorders of the Biliary Tract
Morphine is released into the systemic circulation after epidural administration. Therefore, smooth muscle hypertonicity may result in biliary colic.
DepoDur should be used with caution in patients with biliary tract disease, including acute pancreatitis, as morphine may cause spasm of the sphincter of Oddi and diminish biliary and pancreatic secretions.
Use with Disorders of the Urinary System
The use of epidural opiate analgesia has been associated with disturbances of micturition, especially in males with prostatic hypertrophy. Early recognition of urinary retention and prompt intervention is indicated.
Post-Surgical Ambulation
Patients with reduced circulating blood volume, impaired myocardial function or those receiving sympatholytic drugs should be monitored for the possible occurrence of orthostatic hypotension, a frequent complication in single-dose epidurally administered morphine analgesia.
Intravenous or Intramuscular Administration
Administration of DepoDur via the intravenous or intramuscular routes has not been studied in humans and DepoDur should not be administered by these routes.
Drug Interactions
Local Anesthetics: Administration of DepoDur three minutes after a 3-mL test-dose (lidocaine 1.5% and epinephrine 1:200,000) increases peak serum concentrations of morphine (SeeCLINICAL PHARMACOLOGY). Increasing the interval between the test dose and DepoDur administration to at least 15 minutes minimizes this pharmacokinetic interaction.
Safety and efficacy of DepoDur when used in conjunction with therapeutic epidural doses of lidocaine with epinephrine (for conduction anesthesia) have not been studied in clinical trials.
CNS Depressants: The concurrent use of other central nervous system (CNS) depressants including sedatives, hypnotics, general anesthetics, droperidol, phenothiazines or other tranquilizers, or alcohol increases the risk of respiratory depression, hypotension, profound sedation, or coma. Use with caution and with vigilant monitoring in patients taking these agents.
Carcinogenicity/Mutagenicity/Impairment of Fertility
Studies in animals to evaluate the carcinogenic potential of morphine sulfate have not been conducted. No formal studies to assess the mutagenic potential of morphine have been conducted. In the published literature, the results of in-vitro studies showed that morphine is non-mutagenic in the Drosophila melanogaster lethal mutation assay and produced no evidence of chromosomal aberrations when incubated with murine splenocytes. Contrary to these results, morphine was found to increase DNA fragmentation when incubated in vitro with a human lymphoma cell line. In vivo, morphine has been reported to produce an increase in the frequency of micronuclei in bone marrow cells and immature red blood cells in the mouse micronucleus test and to induce chromosomal aberrations in murine lymphocytes and spermatids. Some of the in‑vivo clastogenic effects reported with morphine in mice may be directly related to increases in glucocorticoid levels produced by morphine in this species.
Pregnancy
Teratogenic Effects (Pregnancy Category C)
No formal studies to assess the teratogenic effects of morphine in animals have been performed. Several literature reports indicate that morphine administered subcutaneously during the early gestational period in mice and hamsters produced neurological, soft tissue and skeletal abnormalities. With one exception, the effects that have been reported were following doses that were maternally toxic and the abnormalities noted were characteristic of those observed when maternal toxicity is present. In one study, following subcutaneous infusion of doses greater than or equal to 0.15 mg/kg in mice, exencephaly, hydronephrosis, intestinal hemorrhage, split supraoccipital, malformed sternebrae, and malformed xiphoid were noted in the absence of maternal toxicity. In the hamster, morphine sulfate given subcutaneously on gestation day eight produced exencephaly and cranioschisis. Morphine was not a significant teratogen in the rat at exposure levels significantly beyond that normally encountered in clinical practice. In one study, however, decreased litter size and viability were observed in the offspring of male rats administered morphine at doses approximately 3-fold the maximum recommended human daily dose (MRHDD) for 10 days prior to mating. In two studies performed in the rabbit, no evidence of teratogenicity was reported at subcutaneous doses up to 100 mg/kg.
In humans, the frequency of congenital anomalies has been reported to be no greater than expected among the children of 70 women who were treated with morphine during the first four months of pregnancy or in 448 women treated with this drug anytime during pregnancy. Furthermore, no malformations were observed in the infant of a woman who attempted suicide by taking an overdose of morphine and other medication during the first trimester of pregnancy.
Nonteratogenic Effects
Published literature indicates that exposure to morphine during pregnancy is associated with reduction in growth and a host of behavioral abnormalities in the offspring of animals. Morphine treatment during gestational periods of organogenesis in rats, hamsters, guinea pigs and rabbits resulted in the following treatment-related embryotoxicity and neonatal toxicity in one or more studies: decreased litter size, embryo-fetal viability, fetal and neonatal body weights, absolute brain and cerebellar weights, lengths or widths at birth and during the neonatal period, delayed motor and sexual maturation, and increased neonatal mortality, cyanosis and hypothermia. Decreased fertility in female offspring, and decreased plasma and testicular levels of luteinizing hormone and testosterone, decreased testes weights, seminiferous tubule shrinkage, germinal cell aplasia, and decreased spermatogenesis in male offspring were also observed. Behavioral abnormalities resulting from chronic morphine exposure of fetal animals included altered reflex and motor skill development, mild withdrawal, and altered responsiveness to morphine persisting into adulthood.
Morphine sulfate should be used by a pregnant woman only if the need for opiate analgesia clearly outweighs the potential risks to the fetus.
Nursing Mothers
In studies of epidural administration of morphine sulfate injection, small amounts of morphine were detected in breast milk. The degree to which morphine sulfate is excreted in human milk following administration of DepoDur has not been studied. Because many drugs are excreted in human milk and because of the potential for adverse reactions in nursing infants from morphine, a decision should be made whether or not to allow nursing during the first 48 hours following DepoDur administration.
Pediatric Use
The safety and effectiveness of DepoDur in pediatric patients below the age of 18 years have not been established and use in this population is not recommended.
Use in the Elderly
DepoDur was studied in clinical trials of 876 subjects; 222 were 65 years of age and older, and 43 of these patients were 75 years of age and over. The efficacy and opiate adverse event profiles in these elderly patients, at the same or lower dose of DepoDur, were similar to those in younger adults (see DOSAGE AND ADMINISTRATION). However, elderly patients (65 years of age or older) may have increased sensitivity to morphine. Comorbid conditions may predispose the elderly population to serious adverse events such as respiratory depression, ileus, hypotension and myocardial infarction.
In general, caution should be exercised in the selection of the dose of DepoDur for an elderly patient. Dosing normally should be at the low end of the range.
DepoDur should be administered to elderly patients (>65 years) after careful evaluation of their underlying medical condition and consideration of the risks associated with DepoDur. Provision for vigilant perioperative monitoring should be arranged for elderly patients receiving DepoDur.
-
ADVERSE EVENTS
In controlled and open label clinical studies with DepoDur, the majority of the adverse events were typical of opiate medications and would be expected in the surgical populations studied. The most common adverse events (greater than 10%) reported at least once during therapy in patients treated with DepoDur were decreased oxygen saturation, hypotension, urinary retention, vomiting, constipation, nausea, pruritus, pyrexia, anemia, headache, and dizziness. Adverse events occurring in 5–10% of study patients were hypoxia, tachycardia, insomnia, and flatulence. Other less common side effects (seen in 2–5% of patients receiving DepoDur) included respiratory depression, hypercapnia, paralytic ileus, somnolence, bladder spasm, abdominal distension, hypoesthesia, hypertension, oliguria, bradycardia, anxiety, back pain, increased sweating, dyspepsia, rigors, dyspnea, hypokalemia, paresthesia, and decreased hematocrit.
Of the patients treated with DepoDur in clinical trials, 4% exhibited signs of respiratory depression requiring treatment with narcotic antagonists. In clinical trials, 90% of respiratory depression occurred within 24 hours after administration of DepoDur. However, onset of respiratory depression occurred in 0.6% of patients after more than 48 hours.
During post-marketing experience, central nervous system (CNS) depression, including obtunded feeling, non-arousable condition, unresponsiveness, confusion, and lethargy, has been reported following epidural administration of DepoDur. In most of these cases with CNS depression, there was concomitant administration of different narcotics or hypnotic/sedative medications in the post-operative period.
During post-marketing experience, severe respiratory depression, involving apnea or respiratory arrest, and cardiac arrest have been reported following administration of labeled doses of DepoDur.
Prolonged respiratory depression or apnea may occur when administration of epidural DepoDur is associated with subarachnoid puncture.
-
DRUG ABUSE AND DEPENDENCE
DepoDur is a μ-agonist opiate and is a Schedule II controlled substance. Morphine, as with other opiates used in analgesia, can be abused and is subject to criminal diversion. Drug addiction is characterized by compulsive use, use for non-medical purposes, and continued use despite harm or risk of harm.
As with all potent μ-agonist opiates, tolerance as well as psychological and physical dependence to morphine may develop irrespective of the route of administration (intravenous, intramuscular, intrathecal, epidural or oral). Tolerance is a condition in which previous exposure to an opiate results in the necessity for increasingly larger doses of the drug in order to produce the same degree of analgesia. Withdrawal symptoms, indicating the presence of psychophysiological dependence, may occur when morphine is discontinued abruptly after chronic administration for analgesia, or upon administration of a drug with full or partial opiate antagonist effects. Care must be taken to avert withdrawal in patients who have been maintained on parenteral/oral opiates prior to epidural administration of DepoDur.
Individuals with a history of opiate or other substance abuse would be considered to be at greater risk of addiction or abuse, given that they are more apt to respond to the euphorigenic and reinforcing properties of morphine. However, concerns about abuse, addiction or diversion of opiates should not prevent proper management of pain.
DepoDur is intended for epidural use only. Abuse of DepoDur may pose a hazard of overdose and death when administered through other routes. This risk is increased with concurrent use of other medications or illicit drugs.
-
OVERDOSAGE
Overdosage of morphine is characterized by respiratory depression, with or without concomitant CNS depression. In severe overdosage, apnea, circulatory collapse, cardiac arrest and death may occur. The conditions that might cause an overdosage situation may vary from patient to patient. A DepoDur dose that is within the labeled dosing guidelines may be found to be more than could be tolerated by an individual patient. During post-marketing experience, spontaneous cases of apnea, respiratory arrest, and cardiac arrest have been reported after administration of labeled DepoDur doses (see WARNINGS and ADVERSE EVENTS). Since respiratory arrest may result either through direct depression of the respiratory center or as the result of hypoxia, attention should primarily be given to the establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Opiates administered in addition to DepoDur to manage pain may precipitate or worsen adverse events such as respiratory depression. Additional opiates should be administered with caution.
The opiate antagonist, naloxone, is a specific antidote. An initial dose of 0.4 to 2 mg of naloxone should be administered intravenously, simultaneously with respiratory resuscitation. If the desired degree of counteraction and improvement in respiratory function is not obtained, naloxone may be repeated at 2- to 3-minute intervals. If no response is observed after 10 mg of naloxone has been administered, the diagnosis of opiate-induced, or partial opiate-induced, toxicity should be questioned. Intramuscular or subcutaneous administration may be used if the intravenous route is not available.
As the duration of effect of naloxone is considerably shorter than that of DepoDur, repeated administration or continuous infusion of naloxone may be necessary. Patients should be closely observed for evidence of recurrence of respiratory depression.
-
DOSAGE AND ADMINISTRATION
DepoDur is intended only for lumbar epidural administration prior to surgery or after clamping of the umbilical cord during cesarean section. DepoDur may be administered via needle or catheter at the lumbar level. Administration of DepoDur at the thoracic level or higher is not recommended because it has not been studied. DepoDur may be administered undiluted or may be diluted up to 5 mL total volume with PRESERVATIVE-FREE 0.9% normal saline.
Vials of DepoDur should be gently inverted to re-suspend the particles immediately prior to withdrawal from the vial. Avoid aggressive agitation. No further reconstitution or dilution is required.
For major orthopedic surgery of the lower extremity the recommended dose of DepoDur is 15 mg. For lower abdominal or pelvic surgery, the recommended dose of DepoDur is 10-15 mg. Some patients may benefit from a 20-mg dose of DepoDur, but the incidence of serious adverse respiratory events was dose-related in clinical trials. For cesarean section, the recommended dose is 10 mg.
DepoDur should be administered to elderly patients (>65 years) after careful evaluation of their underlying medical condition and consideration of the risks associated with DepoDur. Vigilant perioperative monitoring should be exercised for elderly patients receiving DepoDur. In general, as with all opiates, the dose for elderly or debilitated patients should be at the low end of the dosing range.
While DepoDur is indicated for women undergoing cesarean section following clamping of the umbilical cord, DepoDur should not be administered to women for vaginal labor and delivery.
The safety and effectiveness of DepoDur in pediatric patients below the age of 18 years have not been established and use in this population is not recommended.
DepoDur should be administered by or under the direct supervision of a physician experienced in the technique of epidural administration and who is thoroughly familiar with the risks associated with the drug product. It should be administered only in settings where there is adequate patient monitoring. Resuscitative equipment and a specific antagonist (naloxone injection) should be immediately available for the management of respiratory depression. Patient monitoring should be continued for at least 48 hours after dosing, as delayed respiratory depression may occur (see WARNINGS).
Improper placement of a needle or catheter in the epidural space should be ruled out before DepoDur is injected. Techniques to detect improper placement of a needle or catheter include: a) aspiration to check for the presence of blood or cerebrospinal fluid and/or b) administration of a 3-mL test dose of 1.5% PRESERVATIVE-FREE lidocaine and epinephrine (1:200,000). If a test dose is administered, the patient should be observed for tachycardia or sudden onset of segmental anesthesia, indicating that intrathecal injection has occurred. To minimize a pharmacokinetic interaction of DepoDur with the test dose, flush the epidural catheter with 1 mL of PRESERVATIVE-FREE 0.9% normal saline and wait at least 15 minutes after administration of the test dose.
Do not mix DepoDur with any other medications. Once DepoDur has been administered, no other medication should be administered into the epidural space for at least 48 hours.
Do not use an in-line filter during administration of DepoDur.
Although DepoDur is a sterile agent, it does not contain any bacteriostatic agents. Therefore, DepoDur must be administered within 4 hours after withdrawal from the vial. Do not heat-sterilize or gas-sterilize.
Discard any unused portion in a manner appropriate for Schedule II substances.
PROTECT DEPODUR FROM FREEZING. DO NOT ADMINISTER DEPODUR IF IT IS SUSPECTED THAT THE VIAL HAS BEEN FROZEN.
-
SAFETY AND HANDLING INSTRUCTIONS
DepoDur consists of morphine encapsulated in multivesicular lipid-based particles that pose no known risk of handling to healthcare workers. Vials of DepoDur should be gently inverted to re-suspend the particles immediately prior to withdrawal from the vial.
Each vial of DepoDur contains a potent opiate that has been associated with abuse and dependence among health care providers. Appropriate measures should be taken to control this product within the hospital or clinic including rigid accounting, rigorous control of wastage, and restricted access.
Protect DepoDur from freezing. Do not administer DepoDur if it is suspected that the vial has been frozen. Each carton of DepoDur includes a freeze indicator that should be checked prior to administration of the product. Do not administer DepoDur, if the bulb of the freeze indicator has changed from clear to pink or purple, as this indicates that the product may have frozen. Freezing may adversely affect the modified release mechanism of DepoDur.
As a convenience to the hospital pharmacist, each carton of DepoDur includes pharmacy stickers for noting when each vial has been removed from refrigeration and advising to resuspend just prior to use. Following withdrawal from the vial DepoDur may be held at 15° to 30°C (59° to 86°F) for up to 4 hours prior to administration.
-
HOW SUPPLIED
Preservative-free DepoDur (morphine sulfate extended- release liposome injection) is available in 10 mg/mL single-use, amber vials for epidural administration.
10 mg/1 mL vial packaged in cartons of 5 (NDC 24477-020-04)
15 mg/1.5 mL vial packaged in cartons of 5 (NDC 24477-020-05)STORAGE
Protect from freezing. DepoDur should be routinely stored in the refrigerator at 2° to 8°C (36° to 46°F). Keep DepoDur vials in carton during refrigeration. DepoDur may be held at controlled room temperature for up to 30 days in sealed, intact (unopened) vials. Vials stored at controlled room temperature may be separated from the carton, but should not be returned to a refrigerator. Discard vials that have been stored at room temperature for over 30 days
Manufactured for:
EKR Therapeutics, Inc.
Cedar Knolls NJ 07927Manufactured by:
Pacira Pharmaceuticals
San Diego, CA 92121
Copyright © Pacia Pharmaceuticals Inc. 2007
Printed in U.S.A December, 2007
2007-02
-
INGREDIENTS AND APPEARANCE
DEPODUR
morphine sulfate injection, lipid complexProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:24477-020 Route of Administration EPIDURAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength morphine sulfate (UNII: X3P646A2J0) (morphine - UNII:76I7G6D29C) 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength cholesterol (UNII: 97C5T2UQ7J) 3.3 mg in 1 mL DPPG () 0.9 mg in 1 mL tricapryline () 0.3 mg in 1 mL triolein (UNII: O05EC62663) 0.1 mg in 1 mL DOPC () 4.2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24477-020-04 5 in 1 CARTON 1 1 mL in 1 VIAL, SINGLE-USE DEPODUR
morphine sulfate injection, lipid complexProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:24477-020 Route of Administration EPIDURAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength morphine sulfate (UNII: X3P646A2J0) (morphine - UNII:76I7G6D29C) 15 mg in 1.5 mL Inactive Ingredients Ingredient Name Strength cholesterol (UNII: 97C5T2UQ7J) 3.3 mg in 1 mL DPPG () 0.9 mg in 1 mL tricapryline () 0.3 mg in 1 mL triolein (UNII: O05EC62663) 0.1 mg in 1 mL DOPC () 4.2 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24477-020-05 5 in 1 CARTON 1 1.5 mL in 1 VIAL, SINGLE-USE Labeler - Pacira Pharmaceuticals Inc.
