ORAL CLEANSER FIGHTS GUM DISEASE- chlorhexidine digluconate liquid
Swabplus Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
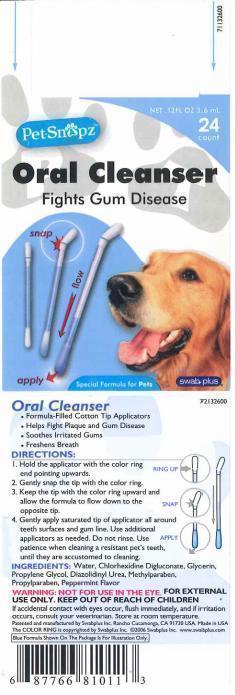
Oral Cleanser Fights Gum Disease
Oral Cleanser
Formula-Filled Cotton Tip Applicators.
Helps Fight Plaque and Gum Disease.
Soothes Irritated Gums.
Freshens Breath.
DIRECTIONS
1. Hold the applicator with the color ring end pointing upwards.
2. Gently snap the tip with the color ring.
3. Keep the tip with the color ring upward and allow the formula to flow down to the opposite tip.
4. Gently apply saturated tip of applicator all around teeth surfaces and gum line. Use additional applicators as needed. Do not rinse. Use patience when cleaning a resistant pet's teeth, until they are accustomed to cleaning.
INACTIVE INGREDIENTS
Water, Chlorhexidine Digluconate (Chlorhexidine Gluconate) Glycerin, Propylene Glycol, Diazolidinyl Urea, Methylparaben, Propylparaben, Peppermint Flavor
STORAGE AND HANDLING
If accidental contact with eyes occur, flush immediately, and if irritation occurs, consult your veterinarian.
Store at room temperature.
| ORAL CLEANSER FIGHTS GUM DISEASE
chlorhexidine digluconate liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Swabplus Inc. (116984439) |