Label: KIDS CHOICE- stannous fluoride gel, dentifrice
-
NDC Code(s):
63783-005-04,
63783-006-04,
63783-007-04,
63783-008-04, view more63783-009-04, 63783-015-04
- Packager: Massco Dental A Division of Dunagin Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
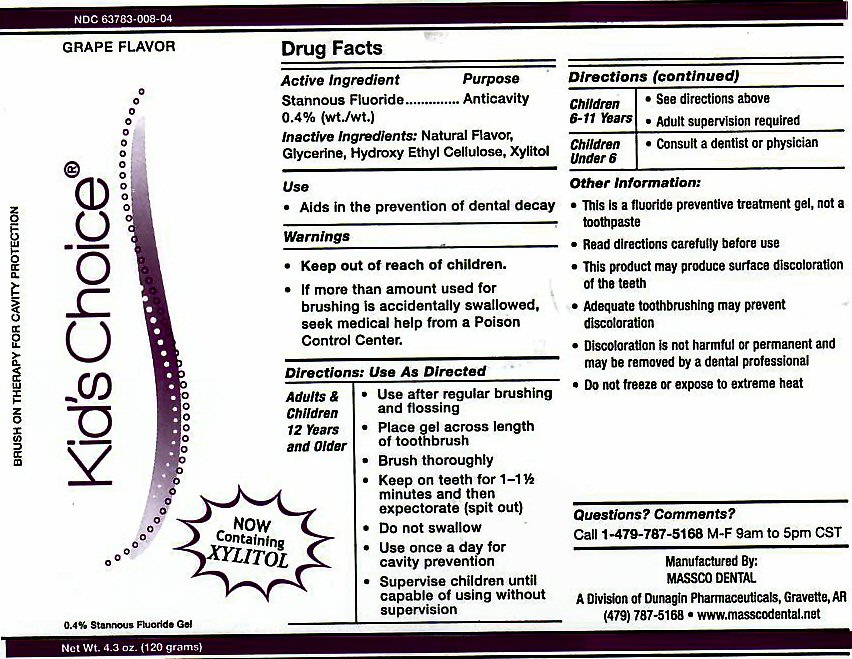
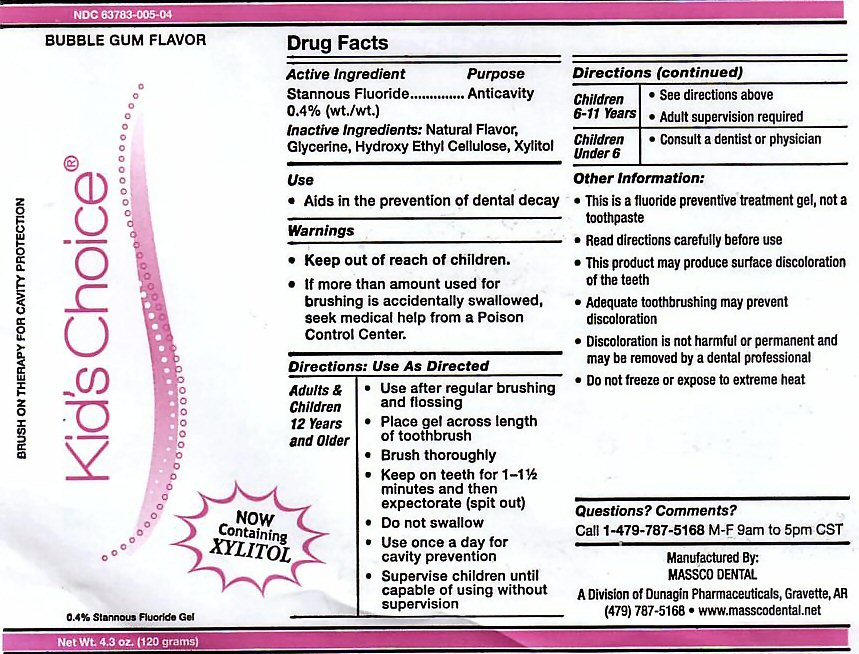
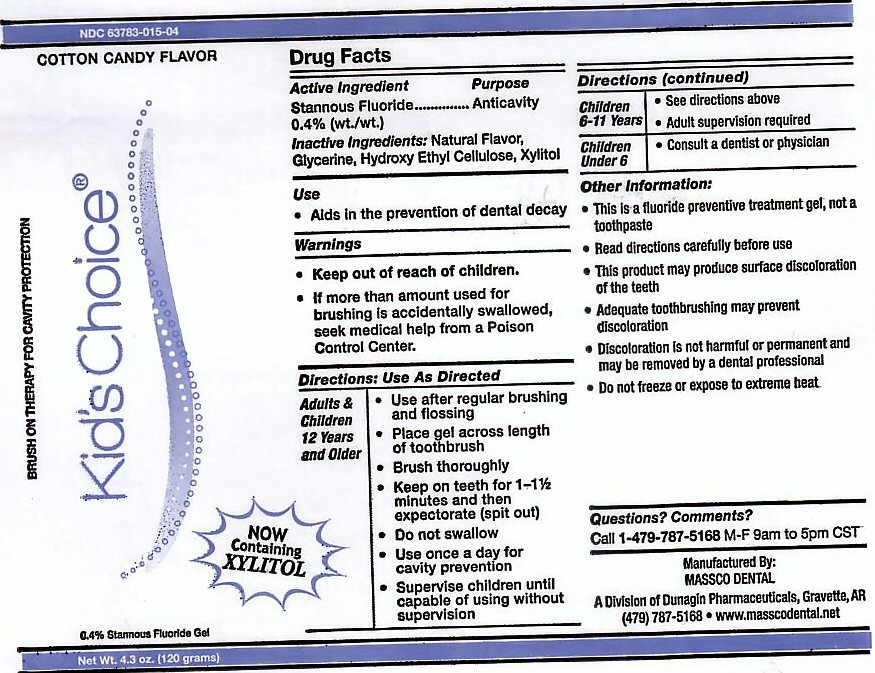
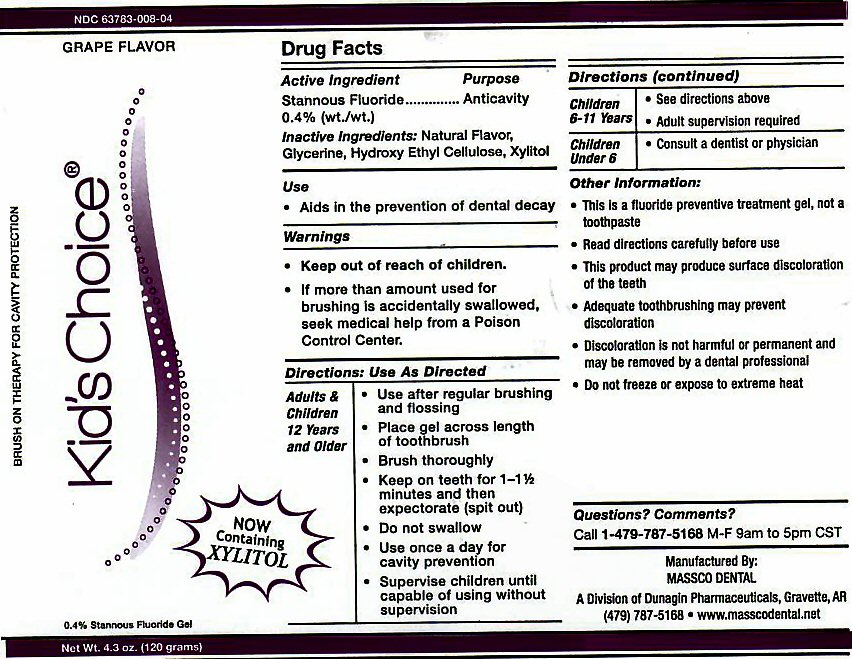
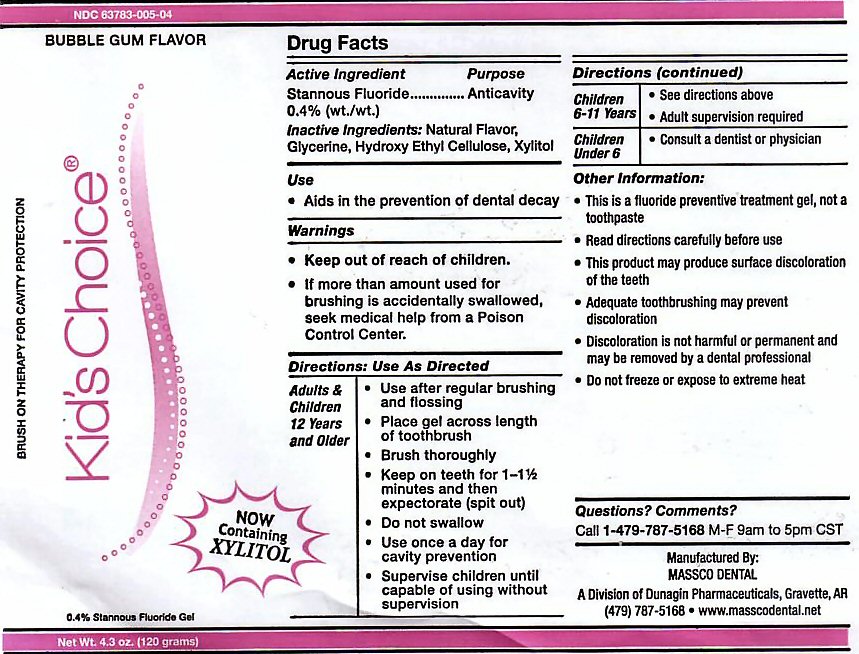
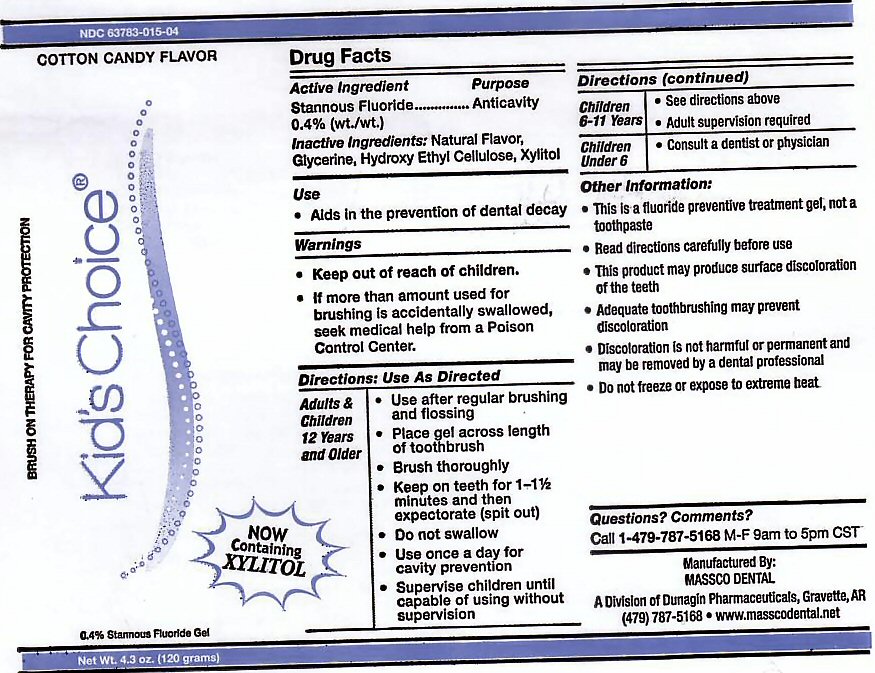
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- USE
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS FOR USE
Adults and Children 12 years and Older: Use after regular brushing and flossing. Place gel across length of toothbrush. Brush Thoroughly. Keep on teeth for 1 - 1 1/2 minutes and then expectorate (spit out). Do not swallow. Use once a day for cavity prevention. Supervise children until capable of using without supervision.
Children 6-11 Years: See directions above. Adult supervision required.
Children Under 6: Consult a Dentist or Physician. -
OTHER INFORMATION
This is a fluoride prevention treatment gel, not a toothpaste. Read directions carefully before use. This product may produce surface discoloration of the teeth. Adequate toothbrushing may prevent discoloration. Discoloration is not harmful or permanent and may be removed by a dental professional. Do not freeze or expose to extreme heat.
- QUESTIONS ? COMMENTS ?
- WARNINGS
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
KIDS CHOICE
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-005 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.12 g in 120 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor BUBBLE GUM (BUBBLE GUM) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-005-04 120 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 KIDS CHOICE
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-006 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.12 g in 120 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor CREME DE MENTHE (HINT OF MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-006-04 120 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 KIDS CHOICE
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.12 g in 120 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor LIME (LIMEAIDE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-007-04 120 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 KIDS CHOICE
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-008 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.12 g in 120 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor GRAPE (GRAPE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-008-04 120 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 KIDS CHOICE
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.12 g in 120 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor CHERRY (CHERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-009-04 120 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 KIDS CHOICE
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63783-015 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.12 g in 120 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor COTTON CANDY (COTTON CANDY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63783-015-04 120 g in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/01/1989 Labeler - Massco Dental A Division of Dunagin Pharmaceuticals (008081858) Registrant - Massco Dental A Division of Dunagin Pharmaceuticals (008081858) Establishment Name Address ID/FEI Business Operations Massco Dental A Division of Dunagin Pharmaceuticals 008081858 manufacture(63783-005, 63783-006, 63783-007, 63783-008, 63783-009, 63783-015)