Label: NMN capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 82042-003-01 - Packager: IDun Bio-Technology Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 8, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- INACTIVE INGREDIENT
-
INDICATIONS & USAGE
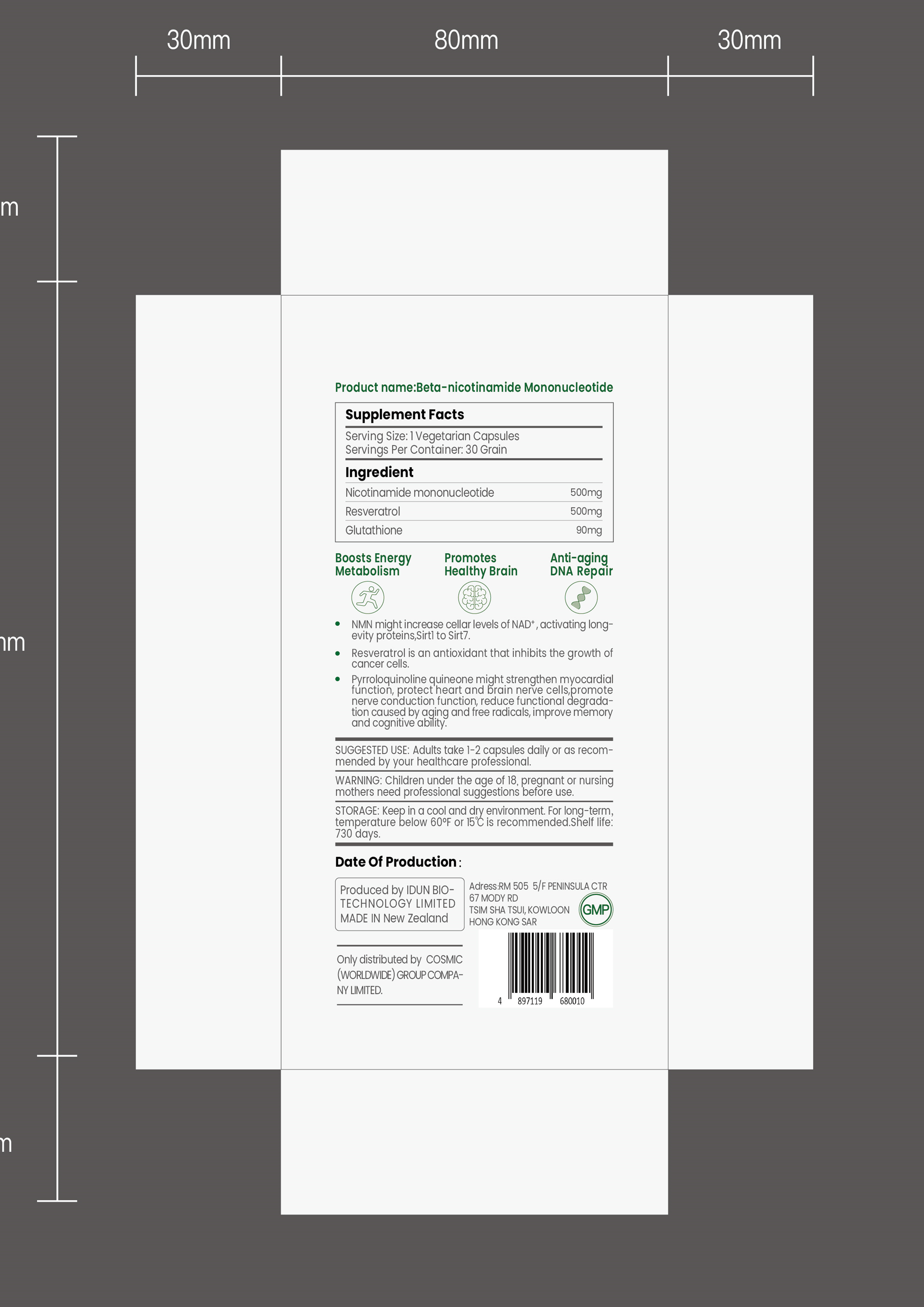
NMN might increase cellar levels of NAD+ , activating long-evity proteins,Sirtl to Sirt7.Resveratrol is an antioxidant that inhibits the growth ofcancer cells.
Pyrroloquinoline quineone might strengthen myocardial function, protect'heart and brain nerve cells,promotenerve conduction function, reduce functional degrada-tion caused by aging and free radicals, improve memoryand cognitive'ability. - SUGGESTED USE
- STORAGE AND HANDLING
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NMN
nmn capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82042-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RESVERATROL (UNII: Q369O8926L) (RESVERATROL - UNII:Q369O8926L) RESVERATROL 45.8 mg in 100 NICOTINAMIDE MONONUCLEOTIDE (UNII: 2KG6QX4W0V) (NICOTINAMIDE MONONUCLEOTIDE - UNII:2KG6QX4W0V) NICOTINAMIDE MONONUCLEOTIDE 45.8 mg in 100 Inactive Ingredients Ingredient Name Strength GLUTATHIONE (UNII: GAN16C9B8O) Product Characteristics Color gray Score 3 pieces Shape OVAL Size 21mm Flavor MALT Imprint Code pretty Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82042-003-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/09/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/09/2021 Labeler - IDun Bio-Technology Limited (655730581) Establishment Name Address ID/FEI Business Operations IDun Bio-Technology Limited 655730581 manufacture(82042-003)