FLUTICASONE PROPIONATE- fluticasone propionate spray, metered
Walgreens
----------
Drug Facts
Uses
Temporarily relieves these symptoms of hay fever or other upper respiratory allergies:
• nasal congestion • runny nose • sneezing • itchy nose
Warnings
Only for use in the nose. Do not spray into your eyes or mouth.
Do not use
- in children under 4 years of age
- to treat asthma
- if you have an injury or surgery to your nose that is not fully healed
- if you have ever had an allergic reaction to this product or any of the ingredients
Ask a doctor or pharmacist before use if you are taking
- medicine for HIV infection (such as ritonavir)
- a steroid medicine for asthma, allergies or skin rash
- ketoconazole pills (medicine for fungal infection)
When using this product
- the growth rate of some children may be slower
- stinging or sneezing may occur for a few seconds right after use
- do not share this bottle with anyone else as this may spread germs
- remember to tell your doctor about all the medicines you take, including this one
Stop use and ask a doctor if
- you have, or come into contact with someone who has, chicken pox, measles or tuberculosis
- your symptoms do not get better within 7 days of starting use or you get new symptoms such as severe facial pain or thick nasal discharge. You may have something more than allergies, such as an infection.
- you get a constant whistling sound from your nose. This may be a sign of damage inside your nose.
- you get an allergic reaction to this product. Seek medical help right away.
- you get new changes to your vision that develop after starting this product
- you have severe or frequent nosebleeds
Directions
- read the Quick Start Guide for how to:
- prime the bottle
- use the spray
- clean the spray nozzle
- shake gently before each use
- use this product only once a day
- do not use more than directed
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER
-
Week 1 - use 2 sprays in each nostril once daily
-
Week 2 through 6 months - use 1 or 2 sprays in each nostril once daily, as needed to treat your symptoms
-
After 6 months of daily use - ask your doctor if you can keep using
CHILDREN 4 TO 11 YEARS OF AGE
-
the growth rate of some children may be slower while using this product. Children should use for the shortest amount of time necessary to achieve symptom relief. Talk to your child's doctor if your child needs to use the spray for longer than two months a year.
-
an adult should supervise use
-
use 1 spray in each nostril once daily
CHILDREN UNDER 4 YEARS OF AGE
-
do not use
Other information
- you may start to feel relief the first day and full effect after several days of regular, once-a-day use
- store at 4° to 30°C (39° to 86°F)
- keep this label and enclosed materials. They contain important additional information.
Inactive ingredients
0.02% w/w benzalkonium chloride, dextrose, microcrystalline cellulose and carboxymethylcellulose sodium, 0.25% w/w phenylethyl alcohol, polysorbate 80, purified water
Questions or comments?
call toll free 1-800-706-5575, weekdays, 8:30am – 5:00pm Eastern Standard Time
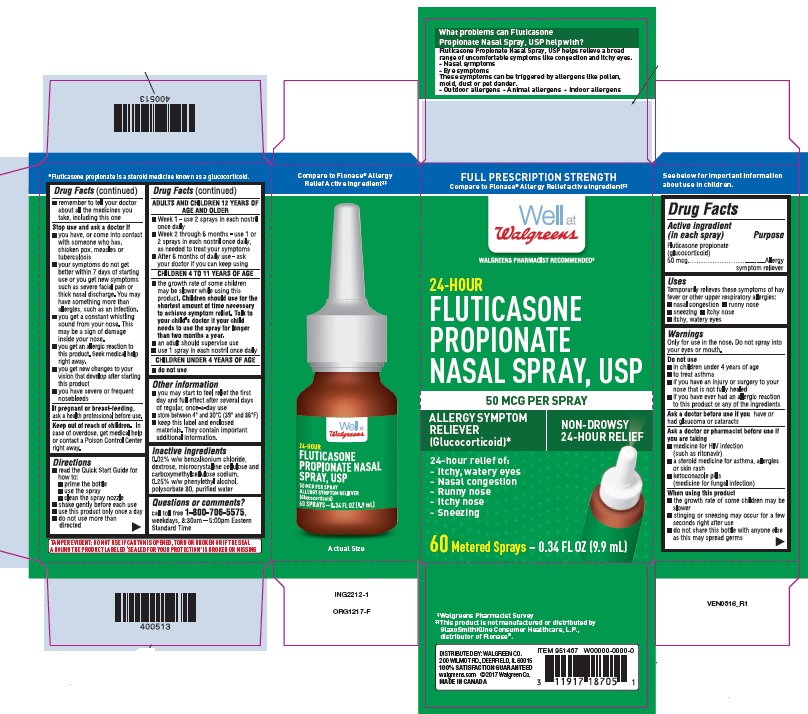
Principal Display Panel - Carton (single)
CARTON LABEL - PRINCIPAL DISPLAY PANEL - 50 mcg per spray
Walgreens NDC 0363-5010-01
Fluticasone Propionate Nasal Spray, USP
120 sprays
Allergy Symptom Reliever Nasal Spray
24 Hour Relief of:
- Nasal Congestion
- Runny Nose
- Itchy Nose
- Sneezing
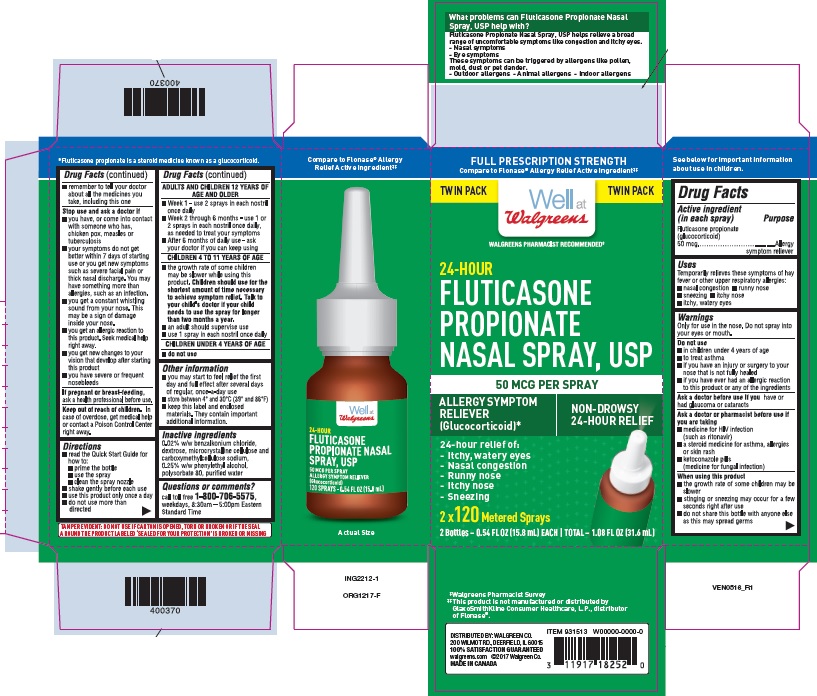
Principal Display Panel - Bottle
BOTTLE LABEL - PRINCIPAL DISPLAY PANEL - 50 mcg per spray
Walgreens NDC 0363-5010-01
Fluticasone Propionate Nasal Spray, USP
120 sprays
Allergy Symptom Reliever Nasal Spray

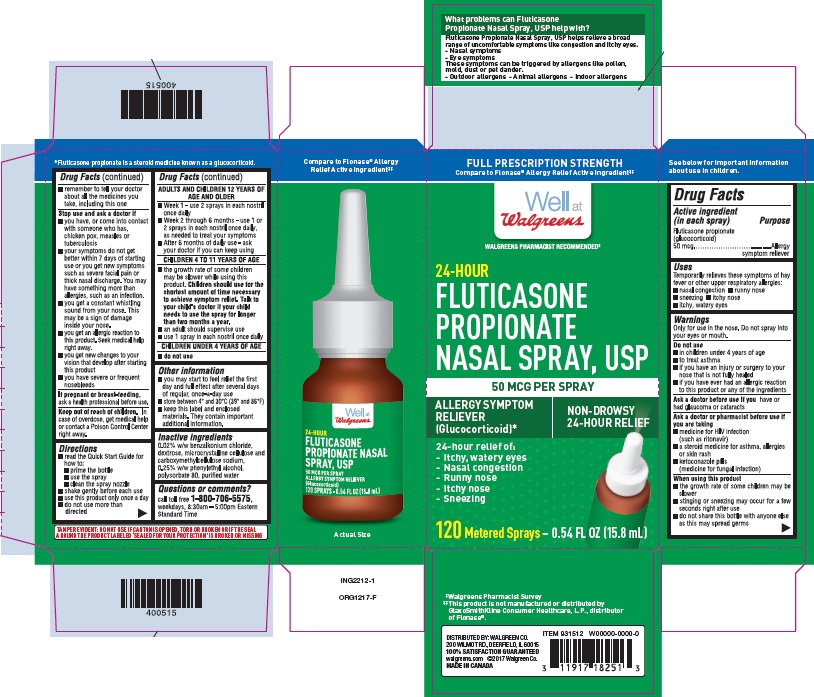
Principle Display Panel - Carton (2x120ms)
CARTON LABEL - PRINCIPAL DISPLAY PANEL - 50 mcg per spray
Walgreens NDC 0363-5010-04
Fluticasone Propionate Nasal Spray, USP
2 x 120 sprays
Allergy Symptom Reliever Nasal Spray
24 Hour Relief of:
- Nasal Congestion
- Runny Nose
- Itchy Nose
- Sneezing
| FLUTICASONE PROPIONATE
fluticasone propionate spray, metered |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Walgreens (008965063) |
| Registrant - Apotex Inc. (209429182) |