Label: ALLERGY RELIEF- levocetirizine dihydrochloride tablet, film coated
- NDC Code(s): 41250-488-01, 41250-488-27
- Packager: Meijer Distribution Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 9, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
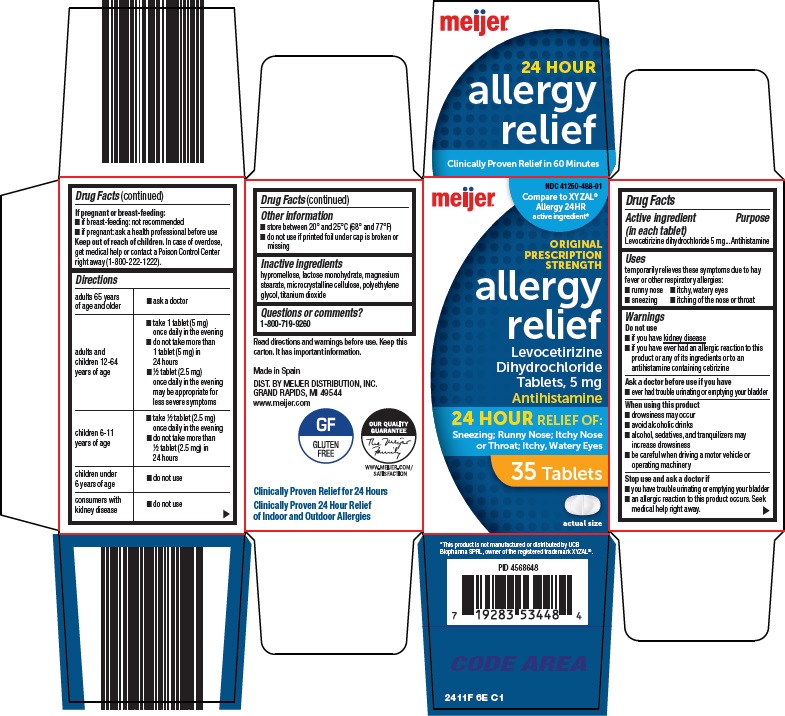
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- •
- if you have kidney disease
- •
- if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing cetirizine
When using this product
- •
- drowsiness may occur
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives, and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- •
- you have trouble urinating or emptying your bladder
- •
- an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
adults 65 years of age and older
- •
- ask a doctor
adults and children 12-64 years of age
- •
- take 1 tablet (5 mg) once daily in the evening
- •
- do not take more than 1 tablet (5 mg) in 24 hours
- •
- ½ tablet (2.5 mg) once daily in the evening may be appropriate for less severe symptoms
children 6-11 years of age
- •
- take ½ tablet (2.5 mg) once daily in the evening
- •
- do not take more than ½ tablet (2.5 mg) in 24 hours
children under 6 years of age
- •
- do not use
consumers with kidney disease
- •
- do not use
- Other information
- Inactive ingredients
- Questions or comments?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
levocetirizine dihydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-488 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOCETIRIZINE DIHYDROCHLORIDE (UNII: SOD6A38AGA) (LEVOCETIRIZINE - UNII:6U5EA9RT2O) LEVOCETIRIZINE DIHYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE Score 2 pieces Shape OVAL Size 8mm Flavor Imprint Code L9CZ;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-488-01 1 in 1 CARTON 02/08/2020 1 35 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:41250-488-27 1 in 1 CARTON 02/06/2020 2 80 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211983 02/06/2020 Labeler - Meijer Distribution Inc (006959555)