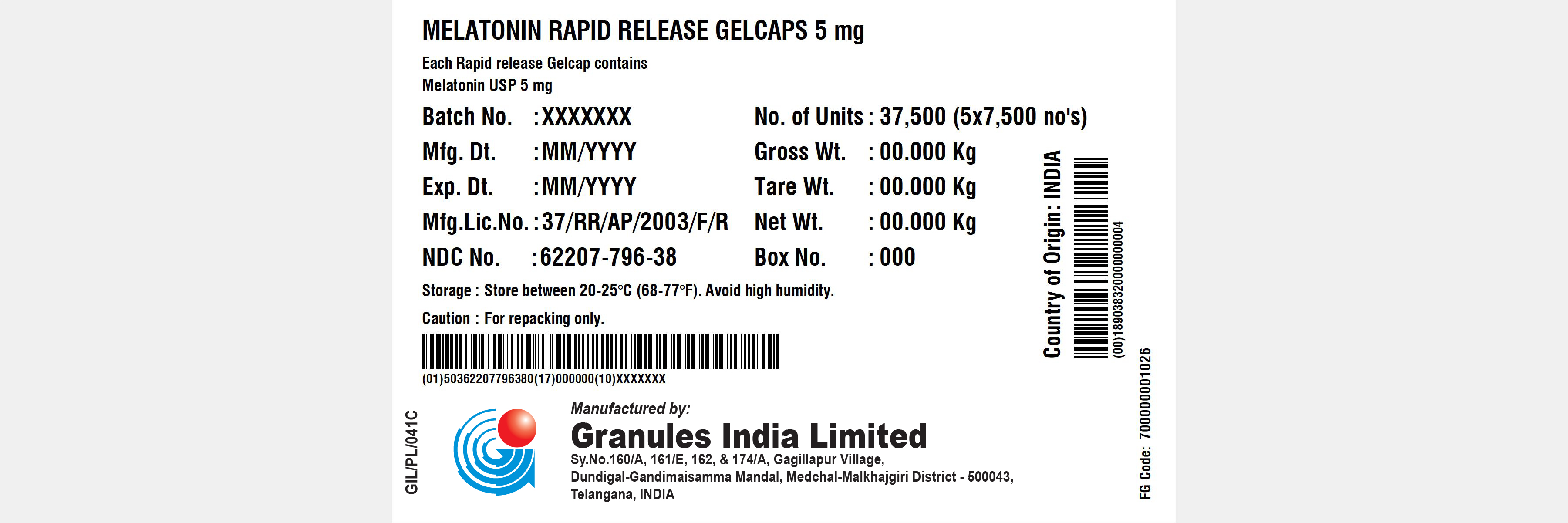
MELATONIN RAPID RELEASE GELCAPS 5 MG- melatonin rapid release gelcaps capsule
Granules India Limited
----------
Melatonin Rapid Release Gelcaps 5mg
| MELATONIN RAPID RELEASE GELCAPS 5 MG
melatonin rapid release gelcaps capsule |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Granules India Limited (915000087) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Granules India Limited | 918609236 | manufacture(62207-796) | |
Revised: 7/2023
Document Id: 01c20743-9c61-0b0d-e063-6294a90a7cd4
Set id: c861b9b5-196f-4439-ab75-247c81055da6
Version: 4
Effective Time: 20230731
Granules India Limited