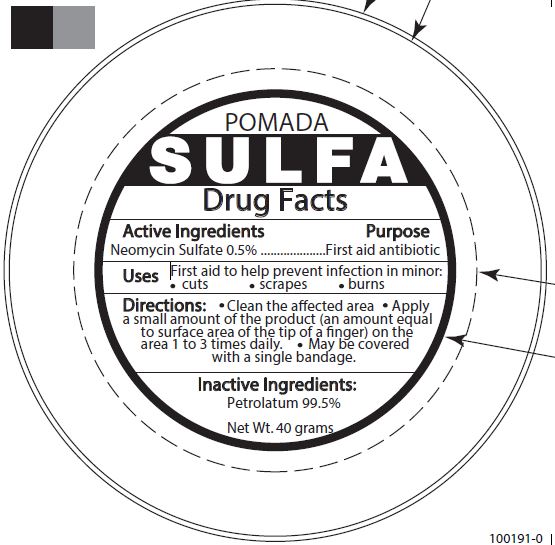
POMADA SULFA FIRST AID ANTIBIOTIC- neomycin sulfate ointment
Grandall Distributing Co., Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Grandall (as PLD) - POMADA SULFA FIRST AID ANTIBIOTIC (48201-103)
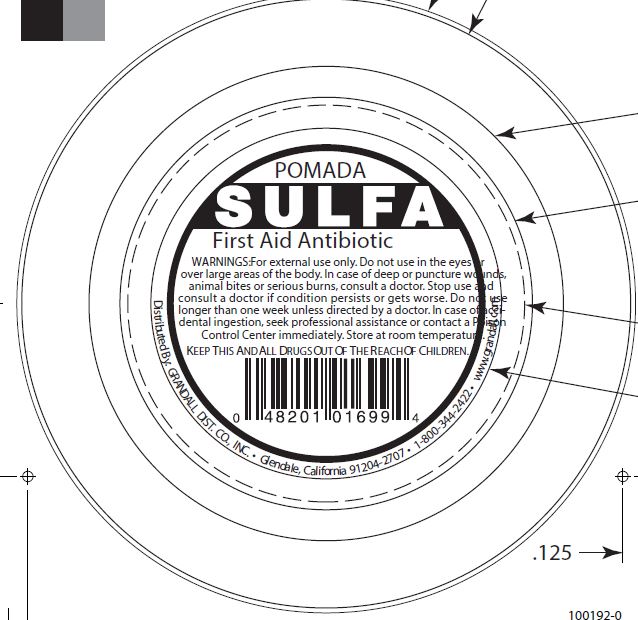
WARNINGS
For external use only. Do not use in the eyes or over large areas of the body. In case of deep or puncture wounds, animal bites or serious burns, consult a doctor. Stop use and consult a doctor if condition persists or gets worse. Do not use longer than one week unless directed by a doctor. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately. Store at room temperature.
| POMADA SULFA FIRST AID ANTIBIOTIC
neomycin sulfate ointment |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Grandall Distributing Co., Inc. (044428324) |
Revised: 8/2023
Document Id: 031f4df4-dcff-63f5-e063-6294a90a3444
Set id: c8181ad8-6e51-4d3f-857c-056821e9aad0
Version: 4
Effective Time: 20230817
Grandall Distributing Co., Inc.