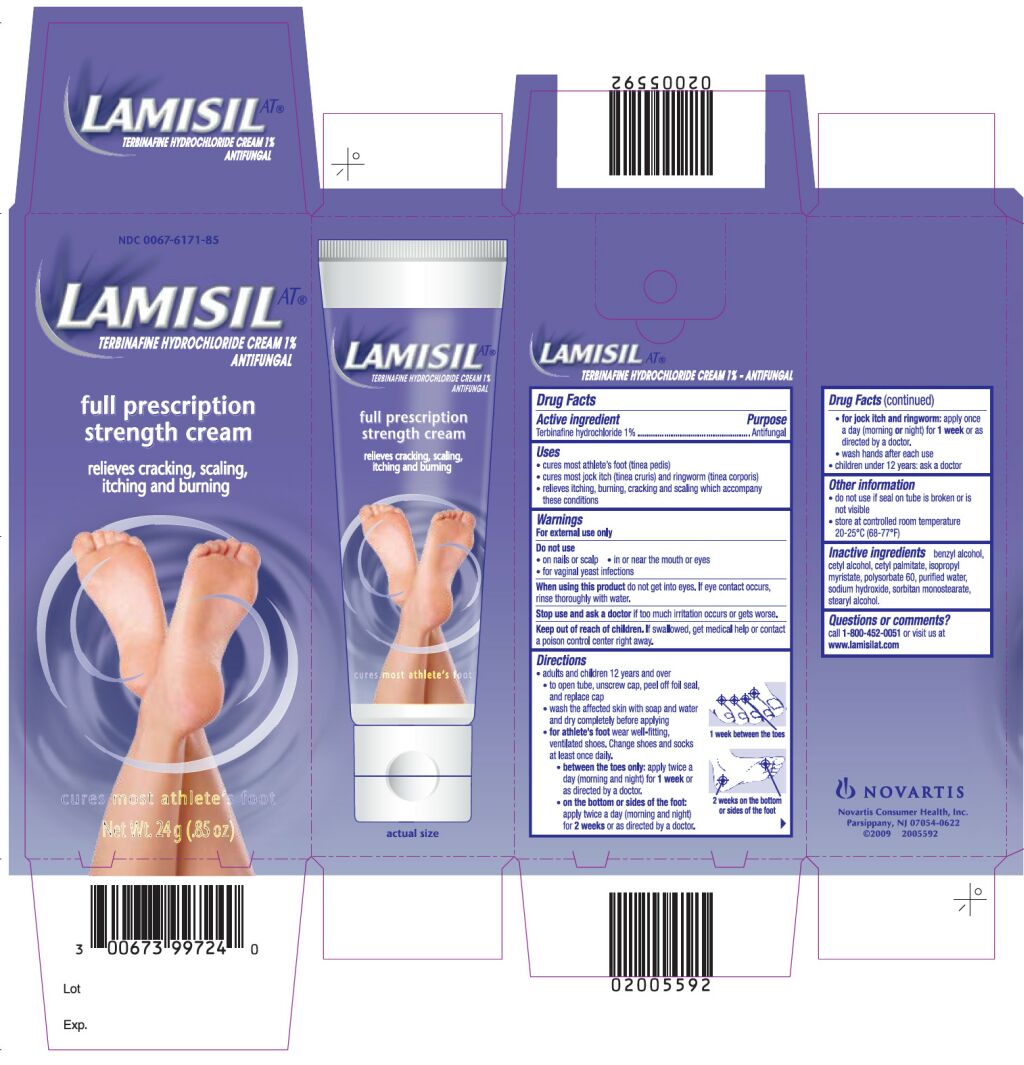
LAMISIL AT- terbinafine hydrochloride cream
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
----------
Drug Facts
Uses
- •
- cures most athlete’s foot (tinea pedis)
- •
- cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- •
- relieves itching, burning, cracking and scaling which accompany these conditions
Keep Out of Reach of Children
If swallowed, get medical help or contact a poison control center right away.
Directions
- •
- adults and children 12 years and over:
- •
- use the tip of the cap to break the seal and open the tube
- •
- wash the affected skin with soap and water and dry completely before applying
- •
-
for athlete’s foot wear well-fitting, ventilated shoes. Change shoes and socks at least once daily.
- •
- between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor
-
-
- •
- on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor.
-
- •
- for jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor.
- •
- wash hands after each use
- •
- children under 12 years: ask a doctor
Other information
- •
- do not use if seal on tube is broken or is not visible
- •
- store at controlled room temperature 20-25°C (68-77°F)
| LAMISIL
AT
terbinafine hydrochloride cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |
Revised: 1/2014
Document Id: d2bf138b-62e5-434a-b86e-d131e4022a06
Set id: c7f36b7b-f205-4de5-b083-acea57281871
Version: 3
Effective Time: 20140101
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC