FUS-SOL- ammonium chloride liquid
Performance Products, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
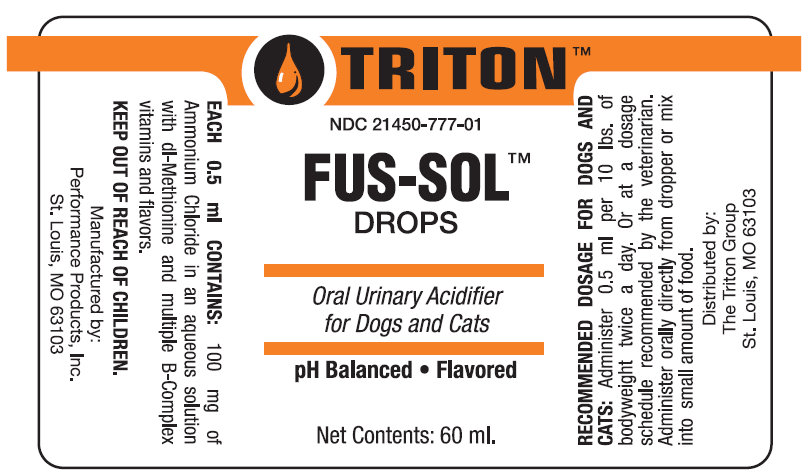
FUS-SOL DROPS
TRITON
Each 0.5 ml CONTAINS: 100 mg of Ammonium Chloride in an aqueous solution with dl-Methionine and multiple B-Complex vitamins and flavors.
KEEP OUT OF REACH OF CHILDREN.
MANUFACTURED BY:
PERFORMANCE PRODUCTS, INC.
ST. LOUIS, MO 63103
RECOMMENDED DOSAGE FOR DOGS AND CATS: Administer 0.5 ml per 10 lbs. of bodyweight twice a day. Or at a a dosage schedule recommended by the veterinarian. Administer orally directly from dropper or mix into amount of food.
DISTRIBUTED BY:
THE TRITON GROUP
ST. LOUIS MO 63103
| FUS-SOL
ammonium chloride liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Performance Products, Inc. (006265888) |
| Registrant - Performance Products, Inc. (006265888) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Performance Products, Inc. | 006265888 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BASF | 014403088 | api manufacture | |