CITRAVET - potassium citrate tablet, chewable
Pegasus Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
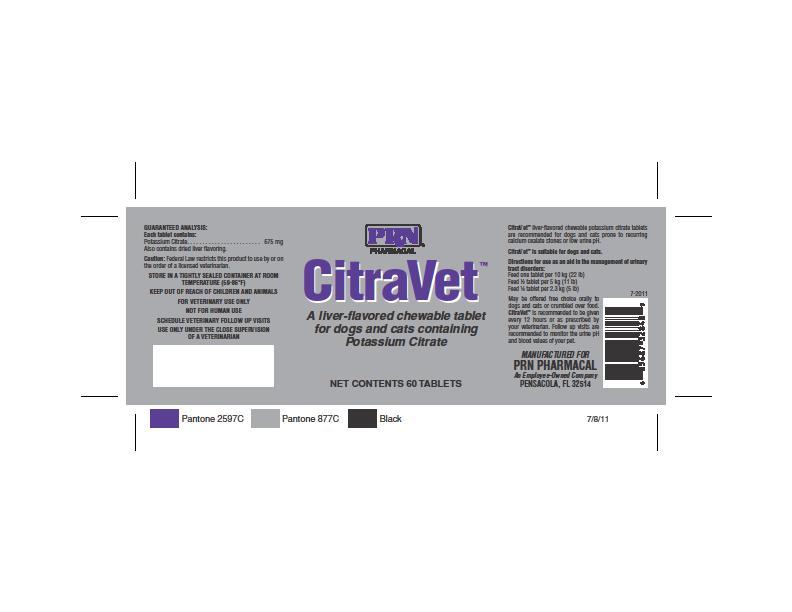
CitraVetTM liver-flavored chewable potassium citrate tablets are recommended for dogs and cats prone to recurring calcium oxalate stones or low urine pH. Citravet TM is suitable for dogs and cats.
Directions for use as an aid in the management of urinary tract disorders:
Feed one tablet per 10 kg (22 lb)
Feed half tablet per 5 kg (11 lb)
Feed quarter tablet per 2.3 kg (5lb)
May be offered free choice orally to the dogs and cats or crumbled over food.
CitraVet TM is recommended to be given every 12 hours or as prescribed by
your veterinarian. Follow up visits are recommended to monitor the urine pH
and blood values of your pet.
GUARANTEED ANALYSIS:
Each tablet contains:
Potassium Citrate 675 mg
Also contains dried liver flavoring
Caution: Federal Law restricts this product to use by or on the order of a licensed veterinarian.
KEEP OUT OF REACH REACH OF CHILDREN AND ANIMALS
FOR VETERINARY USE ONLY
NOT FOR HUMAN USE
SCHEDULE VETERINARY FOLLOW UP VISITS
USE ONLY UNDER THE CLOSE SUPERVISION OF A VETERINARIAN
| CITRAVET
potassium citrate tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pegasus Laboratories, Inc. (108454760) |
| Registrant - Pegasus Laboratories, Inc. (108454760) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pegasus Laboratories, Inc. | 108454760 | manufacture, label, analysis | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Univar USA Inc. | 962683249 | api manufacture | |