INDIGO CARMINE- indigo carmine injection, solution
American Regent, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
INDIGO CARMINE INJECTION (Indigotindisulfonate Sodium Injection, USP)
DESCRIPTION
Each mL contains: Indigotindisulfonate Sodium 8 mg, Water for Injection q.s. pH adjusted, when necessary, with Citric Acid and/or Sodium Citrate. Sterile, nonpyrogenic.
Sufficient Indigo Carmine is contained in each 5 mL ampule to permit accurate withdrawal and administration of the full dose. It gives a deep blue solution when dissolved in water.
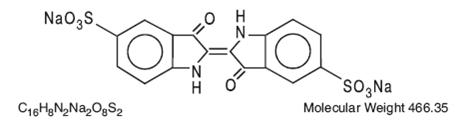
The structural formula is:
CLINICAL PHARMACOLOGY
Indigo Carmine is excreted largely by the kidneys, retaining its blue color during passage through the body.
Elimination of the dye begins soon after injection, appearing in the urine within 10 minutes in average cases. The biological half-life is 4 to 5 minutes following intravenous injection. Larger quantities are necessary when intramuscular injection is employed. Appearance time and elimination are delayed following intramuscular injection.
INDICATIONS AND USAGE
Originally employed as a kidney function test, the chief application of Indigo Carmine at present is localizing ureteral orifices during cystoscopy and ureteral catheterization.
CONTRAINDICATIONS
Indigo Carmine is contraindicated in patients who have previously experienced an adverse reaction following its use.
WARNINGS
An occasional idiosyncratic drug reaction may occur. A mild pressor effect may be encountered in some patients.
PRECAUTIONS
Pregnancy
Animal Reproduction studies have not been conducted with indigotindisulfonate sodium injection. It is also not known whether indigotindisulfonate sodium injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Indigotindisulfonate sodium injection should be given to a pregnant woman only if clearly needed.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Indigo Carmine is administered to a nursing woman.
DRUG ABUSE AND DEPENDENCE
Indigo Carmine is not a controlled substance listed in any of the Drug Enforcement Administration Schedules. Its use is not known to lead to dependence or abuse.
OVERDOSAGE
There are no data available describing the signs, symptoms or laboratory findings accompanying overdosage.
No discernible symptoms of toxicity have been observed in mice with an intravenous dose of 200 mg/kg. After intravenous administration the LD50 was established at 300 mg/kg in mice.
DOSAGE AND ADMINISTRATION
Indigo Carmine solution is injected either by the intravenous or intramuscular route, and its appearance at the ureteral orifices is watched with the cystoscope in place. The intravenous method is preferred because a 5 mL injection is sufficient. A lesser dosage in infants, children and underweight patients will prevent skin coloration.
Since precipitation of indigotindisulfonate sodium may occur, Indigo Carmine Solution must not be diluted prior to injection or injected with infusion assemblies which were used with other solutions.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
NOTE: Since Indigo Carmine is a dark blue solution, visual inspection for particulate matter prior to use may not be possible. To ensure that the withdrawn solution contains no particulates, 5 micron filter straws/filter needles must be used when withdrawing contents of ampules1. The 5 micron nylon mesh filter is suitable for withdrawing the drug product, Indigo Carmine.
1 ASHP Guidelines on Compounding Sterile Preparations
PROTECT FROM LIGHT. Indigo Carmine should be stored in the dark, away from direct light, preferably in the original package.
Store at 20º to 25º C (68º to 77º F); excursions permitted to 15º to 30º C (59º to 86º F) (See USP Controlled Room Temperature).
HOW SUPPLIED
Indigo Carmine Injection
NDC 0517-0375-05 5 mL ampules packaged in boxes of 5
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
IN0375
Rev. 2/17
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 5 mL Container
NDC 0517-0375-01
INDIGO CARMINE
INJECTION
(Indigotindisulfonate Sodium Injection, USP)
0.8% Solution
5 mL AMPULE
FOR IV OR IM USE
Rx Only
AMERICAN
REGENT, INC.
SHIRLEY, NY 11967

PRINCIPAL DISPLAY PANEL – 5 mL Carton
INDIGO CARMINE INJECTION
(Indigotindisulfonate Sodium Injection, USP)
0.8% Solution
NDC 0517-0375-05
5 x 5 mL AMPULES
Rx Only
FOR INTRAVENOUS OR INTRAMUSCULAR USE
Each mL contains: Indigotindisulfonate Sodium 8 mg, Water for Injection q.s. pH adjusted, when necessary, with Citric Acid and/or Sodium Citrate.
Sterile, nonpyrogenic.
WARNING:PROTECT FROM LIGHT. Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15 to 30 C (59 to 86 F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
Rev. 2/17

| INDIGO CARMINE
indigo carmine injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Regent, Inc. (002033710) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| American Regent, Inc. | 002033710 | analysis(0517-0375) , manufacture(0517-0375) | |
