Label: FOLITE- folic acid, magnesium citrate, calcium citrate, vitamin d3, n-acetyl-l-cysteine tablet
- NHRIC Code(s): 50991-977-30
- Packager: Poly Pharmaceuticals, Inc.
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated August 5, 2019
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Description
- Indication and Usage
- Precaution
- Warnings
- Storage
- Dosage and Administration
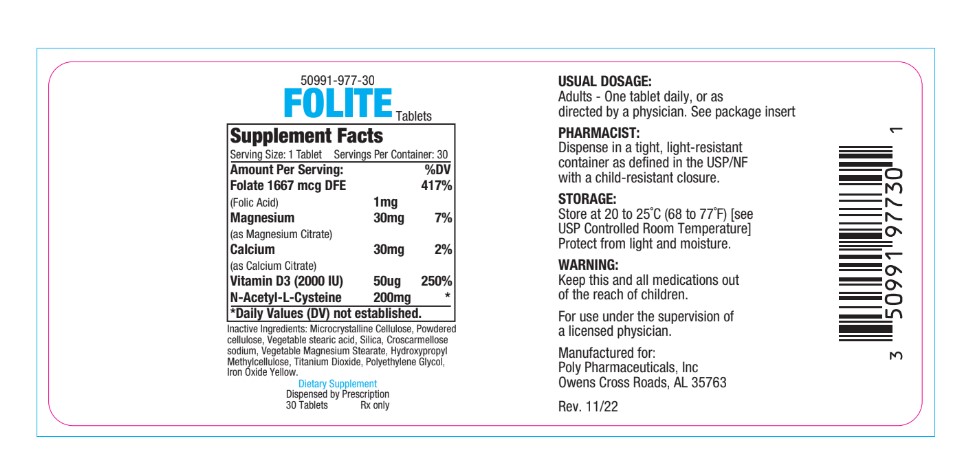
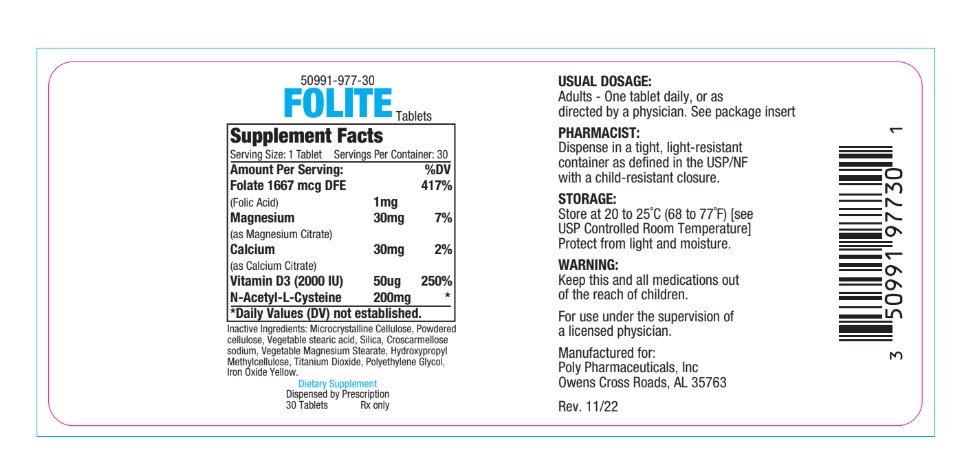
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOLITE
folic acid, magnesium citrate, calcium citrate, vitamin d3, n-acetyl-l-cysteine tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:50991-977 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg Inactive Ingredients Ingredient Name Strength ACETYLCYSTEINE (UNII: WYQ7N0BPYC) 200 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) 50 [iU] CALCIUM CITRATE (UNII: MLM29U2X85) 30 mg MAGNESIUM CITRATE (UNII: RHO26O1T9V) 30 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:50991-977-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 06/01/2019 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value scoring 2 color size (solid drugs) 19 mm shape imprint Labeler - Poly Pharmaceuticals, Inc. (198449894) Registrant - Poly Pharmaceuticals, Inc. (198449894) Establishment Name Address ID/FEI Business Operations Formulation Technology Incorporated 062525910 manufacture