Label: LACTATED RINGERS injection, solution
- NDC Code(s): 13985-803-60
- Packager: MWI (VetOne)
- Category: PRESCRIPTION ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 8, 2014
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
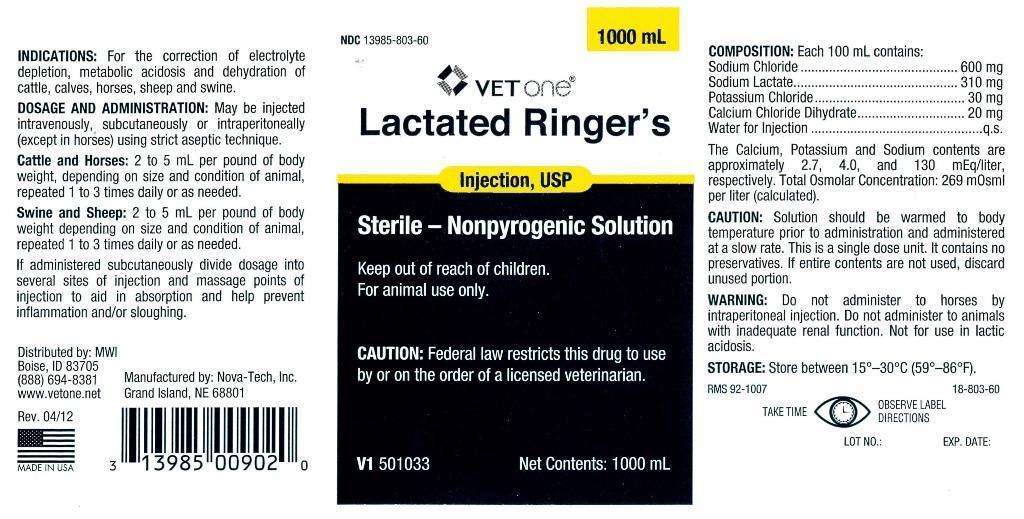
- INDICATIONS:
-
DOSAGE AND ADMINISTRATION:
May be injected intravenously, subcutaneously or intraperitoneally (except in horses) using strict aseptic technique.
Cattle and Horses: 2 to 5 mL per pound of body weight, depending on size and condition of animal, repeated 1 to 3 times daily or as needed.
Swine and Sheep: 2 to 5 mL per pound of body weight depending on size and condition of animal, repeated 1 to 3 times daily or as needed.
If administered subcutaneously divide dosage into several sites of injection and massage points of injection to aid in absorption and help prevent inflammation and/or sloughing.
-
INFORMATION FOR OWNERS/CAREGIVERS
Distributed by: MWI
Boise, ID 83705
(88) 694-8381
www.vetone.netManufactured by: Nova-Tech, Inc.
Grand Island, NE 68801Rev. 04/12
V1 501033
Net Contents: 1000 mL
RMS 92-1007
18-803-60
LOT NO.: EXP. DATE:
- VETERINARY INDICATIONS
- KEEP OUT OF REACH OF CHILDREN
- CAUTION:
-
COMPOSITION:
Each 100 mL contains:
Sodium Chloride........................600 mg
Sodium Lactate.........................310 mg
Potassium Chloride.....................30 mg
Calcium Chloride Dihydrate..........20 mg
Water for Injection.........................q.s.The Calcium, Potassium and Sodium contents are approximately 2.7, 4.0, and 130 mEq/liter, respectively. Total Osmolar Concentration: 269 mOsml per liter (calculated).
- CAUTION:
- WARNING:
- STORAGE:
- PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LACTATED RINGERS
lactated ringers injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-803 Route of Administration INTRAVENOUS, SUBCUTANEOUS, INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 600 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (LACTIC ACID - UNII:33X04XA5AT) SODIUM LACTATE 310 mg in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CATION 30 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CHLORIDE 20 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-803-60 1000 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/30/2012 Labeler - MWI (VetOne) (019926120) Registrant - MWI (VetOne) (019926120) Establishment Name Address ID/FEI Business Operations Nova-Tech, Inc. 196078976 manufacture