Label: CALCIUM CHLORIDE injection, solution
- NDC Code(s): 0409-1631-10, 0409-1631-40, 0409-4928-34
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use 10% CALCIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for 10% CALCIUM CHLORIDE INJECTION.
CALCIUM CHLORIDE injection, for intravenous use only
Initial U.S. Approval: 1938INDICATIONS AND USAGE
Calcium Chloride Injection is a form of calcium indicated for the treatment of adult and pediatric and patients with acute symptomatic hypocalcemia. (1)
Limitations of Use:
The safety and effectiveness of Calcium Chloride Injection for long-term use has not been established.
DOSAGE AND ADMINISTRATION
- •
- Administer Calcium Chloride Injection by slow intravenous infusion (not to exceed 1 mL/minute), in a central or deep vein. (2.1)
- •
- Do not use intramuscularly or subcutaneously. (2.1)
- •
- Do not administer unless solution is clear and seal is intact. (2.1)
- •
- Stop the administration if the patient complains of any administration-related discomfort, it may be resumed when symptoms disappear. (2.1)
- •
- The recommended adult dose is from 200 mg to 1,000 mg. (2.2)
- •
- The recommended pediatric dose is from 2.7 to 5 mg/kg of calcium chloride. (2.2)
- •
- Repeated injections may be required because of rapid calcium excretion. (2.2)
- •
- See the full prescribing information for the recommended starting dose in patients with renal impairment. (2.3)
- •
- Do not mix Calcium Chloride Injection with ceftriaxone or administer these products simultaneously via a Y-site because concurrent use can lead to the formation of ceftriaxone-calcium precipitates. (2.4)
DOSAGE FORMS AND STRENGTHS
Calcium Chloride Injection, USP (single-dose) is supplied as: (3)
- •
- 10% (1,000 mg/10 mL) (100 mg/mL) in an Ansyr® Plastic Syringe
- •
- 10% (1,000 mg/10 mL) (100 mg/mL) in a LifeShield® Abboject® Glass Syringe
The 100 mg/mL concentration represents 27 mg or 1.4 mEq of elemental calcium per mL of solution. (3)
CONTRAINDICATIONS
Calcium Chloride Injection is contraindicated in:
- •
- Patients with ventricular fibrillation. (4)
- •
- Patients with asystole and electromechanical dissociation. (4)
- •
- Newborns (up to 28 days of age) if they require (or are expected to require) ceftriaxone intravenous treatment, regardless of whether these products would be received at different times or through separate intravenous lines. (4, 5.1)
WARNINGS AND PRECAUTIONS
- •
- End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates: Calcium Chloride Injection is contraindicated in newborns (up to 28 days of age) if they require (or are expected to require) ceftriaxone intravenous treatment. In patients older than 28 days of age, do not mix or administer simultaneously with ceftriaxone intravenous solutions, even via different infusion lines or at different infusion sites as it can lead to precipitation of ceftriaxone-calcium. (5.1)
- •
- Hypotension, Bradycardia, Arrhythmias, and Syncope with Rapid Administration: Too rapid an injection exceeding 1 mL/minute may lead to hypotension and syncope. (2.1, 5.2)
- •
- Arrhythmias with Concomitant Digoxin Use: Avoid use of Calcium Chloride Injection in patients receiving digoxin. Closely monitor ECG and calcium levels if concomitant therapy is necessary. (5.3, 7.1)
- •
- Tissue Necrosis and Calcinosis: Administer Calcium Chloride Injection slowly through a small needle into a large vein to minimize the risk of tissue necrosis, ulceration and calcinosis. Avoid extravasation or accidental injection into perivascular tissues. Immediately discontinue administration should perivascular infiltration occur. (2.1, 5.4)
- •
- Aluminum Toxicity: Risk of toxicity with prolonged administration if kidney function is impaired. Premature neonates are particularly at risk. When prescribing Calcium Chloride Injection in patients receiving parenteral nutrition solutions, limit the total daily patient exposure to aluminum to no more than 5 mcg/kg/day. (5.5)
ADVERSE REACTIONS
Adverse reactions have included paraesthesia (upon rapid injection), calcium taste, sense of oppression, sense of “heat wave”, local burning sensation, injection site extravasation, injection site reactions, peripheral vasodilatation, and decreased blood pressure. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Digoxin: Avoid concomitant use with Calcium Chloride Injection. If concomitant use is unavoidable, monitor ECG closely during administration of Calcium Chloride Injection. (5.3, 7.1)
- •
- Calcium Channel Blockers: Avoid concomitant use with Calcium Chloride Injection. If concomitant use is unavoidable, monitor blood pressure closely during administration of Calcium Chloride Injection. (7.2)
- •
- Drugs That Increase the Risk of Hypercalcemia: Increase the frequency of calcium concentration monitoring in patients taking Calcium Chloride Injection concomitantly with other drugs that increase the risk of hypercalcemia. (7.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage and Administration
2.3 Recommended Starting Dose in Patients with Renal Impairment
2.4 Drug Incompatibilities
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates
5.2 Hypotension, Bradycardia, Arrhythmias, and Syncope with Rapid Administration
5.3 Arrhythmias with Concomitant Digoxin Use
5.4 Tissue Necrosis and Calcinosis
5.5 Aluminum Toxicity
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Digoxin
7.2 Calcium Channel Blockers
7.3 Drugs That Increase the Risk of Hypercalcemia
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Administer Calcium Chloride Injection by slow intravenous infusion in a central or deep vein in adults and pediatric patients (with or without renal impairment); do not administer by bolus [see Warnings and Precautions (5.2, 5.4)]. The maximum recommended infusion rate is 1 mL/minute (100 mg/minute).
Additional important administration instructions regarding Calcium Chloride Injection are as follows:
- •
- Do not use intramuscularly or subcutaneously to avoid tissue necrosis calcinosis cutis [see Warnings and Precautions (5.4)].
- •
- Visually inspect for particulate matter and discoloration prior to administration (the solution is clear, and the seal is intact). Do not administer if the solution is unclear or the seal is not intact.
- •
- Stop the administration if the patient complains of any administration-related discomfort; administration may be resumed when symptoms disappear.
- •
- Discard the unused portion.
- •
- If time permits, allow the solution to warm to body temperature.
2.2 Recommended Dosage and Administration
The recommended dose range of Calcium Chloride Injection in:
- •
- Adults is from 200 mg to 1,000 mg.
- •
- Pediatric patients is from 2.7 to 5 mg/kg of calcium chloride.
Dosing of this Calcium Chloride Injection product is not possible in patients who require doses less than 200 mg because the recommended dose cannot be achieved with the supplied syringe. For patients who require doses less than 200 mg, use another calcium chloride injection product that allows dosing of less than 200 mg.
Individualize the dose for a patient within these dose ranges depending on serum ionized calcium level, severity of hypocalcemia symptoms, and the acuity of hypocalcemia onset.
Repeated injections may be required because of rapid excretion of calcium.
2.3 Recommended Starting Dose in Patients with Renal Impairment
The recommended starting dose of Calcium Chloride Injection in [see Use in Specific Populations (8.6)]:
- •
- Adults with renal impairment is 200 mg.
- •
- Pediatric patients is 2.7 mg/kg of calcium chloride.
2.4 Drug Incompatibilities
Do not mix Calcium Chloride Injection with other drugs simultaneously. Do not mix Calcium Chloride Injection with ceftriaxone or administer these products simultaneously via a Y-site because concurrent use can lead to the formation of ceftriaxone-calcium precipitates [see Warnings and Precautions (5.1)]:
- •
- In neonates (28 days of age or younger), concomitant use of Calcium Chloride Injection and ceftriaxone is contraindicated [see Contraindications (4)].
- •
- In patients older than 28 days of age, ceftriaxone and calcium-containing products may be administered sequentially, provided the infusion lines are thoroughly flushed between infusions with a compatible fluid.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Calcium Chloride Injection is contraindicated in:
- •
- Patients with ventricular fibrillation
- •
- Patients with asystole and electromechanical dissociation
Newborns (up to 28 days of age) if they require (or are expected to require) ceftriaxone intravenous treatment because of the risk of precipitation of ceftriaxone-calcium, regardless of whether these products would be received at different times or through separate intravenous lines [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates
The use of Calcium Chloride Injection is contraindicated in newborns (up to 28 days of age) if they require (or are expected to require) ceftriaxone intravenous treatment because of the risk of precipitation of ceftriaxone-calcium, regardless of whether these products would be received at different times or through separate intravenous lines [see Contraindications (4)]. Cases of fatal reactions with calcium-ceftriaxone precipitates in lungs and kidneys in premature and full-term newborns aged less than 1 month have occurred when ceftriaxone and calcium were administered either simultaneously or non-simultaneously and through different intravenous lines. In-vitro studies demonstrated that neonates have an increased risk of precipitation of ceftriaxone-calcium compared to other age groups.
In patients older than 28 days of age, Calcium Chloride Injection and ceftriaxone intravenous solutions may be administered sequentially one after another if infusion lines at different sites are used, infusion lines are replaced, or infusion lines are thoroughly flushed between infusions with physiological salt solution to avoid precipitation. Do not mix or administer Calcium Chloride Injection simultaneously with ceftriaxone, even if using different infusion lines or different infusion sites as it can lead to precipitation of ceftriaxone-calcium [see Dosage and Administration (2.4)].
5.2 Hypotension, Bradycardia, Arrhythmias, and Syncope with Rapid Administration
Rapid injection of Calcium Chloride Injection may cause vasodilation, decreased blood pressure, bradycardia, arrhythmias, syncope, and cardiac arrest. It is particularly important to prevent a high concentration of calcium from reaching the heart because of the risk of syncope. Too rapid an injection exceeding 1 mL/minute may lead to hypotension and cardiac syncope [see Dosage and Administration (2.1)].
5.3 Arrhythmias with Concomitant Digoxin Use
Arrhythmias may occur if Calcium Chloride Injection and digoxin are administered together. Hypercalcemia resulting from an overdose of Calcium Chloride Injection increases the risk of digoxin toxicity. Avoid the use of Calcium Chloride Injection in patients receiving digoxin. If concomitant therapy is necessary, closely monitor ECG and calcium levels [see Drug Interactions (7.1)].
5.4 Tissue Necrosis and Calcinosis
Administration of Calcium Chloride Injection in patients with local trauma may result in calcinosis cutis due to transient increase in local calcium concentration. Calcinosis cutis can occur with or without extravasation of Calcium Chloride Injection, is characterized by abnormal dermal deposits of calcium salts, and clinically manifests as papules, plaques, or nodules that may be associated with erythema, swelling, or induration. Tissue necrosis, ulceration, and secondary infection are the most serious complications.
To minimize the risk of tissue necrosis, ulceration and calcinosis, administer Calcium Chloride Injection slowly through a small needle into a large vein [see Dosage and Administration (2.1)]. Avoid extravasation or accidental injection into perivascular tissues. Should perivascular infiltration occur, immediately discontinue intravenous administration at that site and treat as needed.
5.5 Aluminum Toxicity
Calcium Chloride Injection contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Research indicates that patients with impaired kidney function, including premature (preterm) neonates and preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day can accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower amounts of aluminum.
Exposure to aluminum from Calcium Chloride Injection at the recommended dose is not more than 10 mcg [see Dosage and Administration (2.2) and Description (11)]. When prescribing Calcium Chloride Injection in patients receiving parenteral nutrition solutions, limit the total daily patient exposure to aluminum to no more than 5 mcg/kg/day.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are also described elsewhere in the labeling:
- •
- End-Organ Damage due to Intravascular Ceftriaxone-Calcium Precipitates [see Warnings and Precautions (5.1)]
- •
- Hypotension, Bradycardia, Arrhythmias, and Syncope with Rapid Administration [see Warnings and Precautions (5.2)]
- •
- Arrhythmias with Concomitant Digoxin Use [see Warnings and Precautions (5.3)]
- •
- Tissue Necrosis and Calcinosis [see Warnings and Precautions (5.4)]
- •
- Aluminum toxicity [see Warnings and Precautions (5.5)]
The following adverse reactions have been identified in literature and postmarketing reports of calcium chloride. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- •
- Nervous system disorders: Paraesthesia (upon rapid injection), calcium taste
- •
- General disorders and administration site conditions: Sense of oppression, sense of “heat wave”, local burning sensation, injection site extravasation, injection site reactions
- •
- Cardiovascular disorders: Peripheral vasodilatation, decreased blood pressure
-
7 DRUG INTERACTIONS
7.1 Digoxin
Avoid the concomitant use of Calcium Chloride Injection with digoxin. If concomitant use is unavoidable, monitor ECG closely during administration of Calcium Chloride Injection.
Synergistic arrhythmias may occur with concomitant use. The use of Calcium Chloride Injection may result in hypercalcemia which increases the risk of digoxin toxicity [see Warnings and Precautions (5.3)].
7.2 Calcium Channel Blockers
Concomitant use of Calcium Chloride Injection and calcium channel blockers may reduce the response to calcium channel blockers. Avoid concomitant use. If concomitant use is unavoidable, monitor blood pressure closely during administration of Calcium Chloride Injection.
7.3 Drugs That Increase the Risk of Hypercalcemia
Increase frequency of monitoring of calcium concentrations in patients taking concomitant Calcium Chloride Injection and other drugs that increase the risk of hypercalcemia (e.g., calcipotriene, estrogen, lithium, parathyroid hormone, teriparatide, thiazide diuretics, Vitamin A, and Vitamin D).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Administration of Calcium Chloride Injection for the treatment of acute symptomatic hypocalcemia during pregnancy is not expected to cause major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the mother and the fetus associated with development of hypocalcemia during pregnancy (see Clinical Considerations). Animal reproduction studies have not been conducted with Calcium Chloride Injection.
The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal/Neonatal Risk
Maternal hypocalcemia can result in an increased rate of spontaneous abortion, premature and dysfunctional labor, and possibly preeclampsia. Infants born to mothers with hypocalcemia can develop fetal and neonatal hyperparathyroidism, which in turn can cause fetal and neonatal skeletal demineralization, subperiosteal bone resorption, osteitis fibrosa cystica and neonatal seizures.
8.2 Lactation
Risk Summary
Calcium is present in human milk. Administration of the approved recommended dose of Calcium Chloride Injection to the mother is not expected to cause harm to a breastfed infant. There is no information on the effects of Calcium Chloride Injection on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Calcium Chloride Injection and any potential adverse effects on the breastfed infant from Calcium Chloride Injection or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Calcium Chloride Injection for the treatment of acute symptomatic hypocalcemia have been established in pediatric patients.
The use of Calcium Chloride Injection is contraindicated in newborns if they require (or are expected to require) ceftriaxone intravenous treatment because of the risk of precipitation of ceftriaxone-calcium, regardless of whether these products would be received at different times or through separate intravenous lines [see Contraindications (4) and Warnings and Precautions (5.1)].
In pediatric patients older than 28 days of age, Calcium Chloride Injection and ceftriaxone intravenous solutions may be administered sequentially one after another if infusion lines at different sites are used, infusion lines are replaced, or infusion lines are thoroughly flushed between infusions with physiological salt solution to avoid precipitation. Do not mix or administer Calcium Chloride Injection simultaneously with ceftriaxone, even if using different infusion lines or different infusion sites as it can lead to precipitation of ceftriaxone-calcium.
Calcium Chloride Injection contains aluminum that may be associated with central nervous system and bone toxicity. Because of immature renal function, preterm infants receiving prolonged parenteral nutrition treatment with Calcium Chloride Injection may be at higher risk of aluminum toxicity [see Warnings and Precautions (5.2)].
8.5 Geriatric Use
Clinical studies of Calcium Chloride Injection did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
8.6 Renal Impairment
The use of Calcium Chloride Injection in patients with renal impairment may increase the risk of a higher calcium-phosphorus product. For patients with renal impairment, initiate Calcium Chloride Injection at the lowest recommended dose within the recommended dose range [see Dosage and Administration (2.2)]. Monitor serum calcium levels frequently based on the severity of the renal impairment and the risk of a high calcium-phosphorus product (e.g., every 4 hours).
-
10 OVERDOSAGE
Overdosage of Calcium Chloride Injection may lead to hypercalcemia. Symptoms of hypercalcemia typically develop when the total serum calcium concentration is ≥12 mg/dL, and include shortening of QT interval, bradycardia, hypertension, anorexia, nausea, vomiting, bowel hypomotility and constipation, muscle weakness, bone pain, decreased concentration, depression, weakness, fatigue, confusion, hallucinations, disorientation, hypotonicity, seizures, and coma. Hypercalcemia effects on kidney include diminished ability to concentrate urine and diuresis.
In the event of overdosage, promptly discontinue Calcium Chloride Injection , the patient should be re-evaluated and appropriate countermeasures should be instituted, if necessary [see Warnings and Precautions (5), Adverse Reactions (6)].
-
11 DESCRIPTION
10% Calcium Chloride Injection, USP is a sterile, nonpyrogenic, hypertonic solution for single administration only. Each mL contains 100 mg (1.4 mEq/mL) of calcium chloride, dihydrate (1.4 mEq each of Ca++ and Cl-) in water for injection. It is provided in a 10 mL single-dose syringe for intravenous injection. The solution contains no bacteriostat, antimicrobial agent or added buffer. The pH of 10% Calcium Chloride Injection, USP is 5.5 to 7.5 when diluted with water for injection to make a 5% solution. May contain hydrochloric acid and/or sodium hydroxide for pH adjustment. The osmolar concentration is 2.04 mOsmol/mL (calc.). 10% Calcium Chloride Injection, USP is oxygen sensitive.
Calcium Chloride, USP dihydrate is chemically designated CaCl2 • 2H2O (dihydrate) and is described as white, odorless fragments or granules freely soluble in water.
Calcium Chloride Injection, USP contains no more than 1,000 mcg/L of aluminum [see Warnings and Precautions (5.2)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Intravenous administration of calcium chloride increases serum ionized calcium concentration. Calcium chloride dissociates into ionized calcium in plasma.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of Calcium Chloride Injection have not been fully characterized.
12.3 Pharmacokinetics
Absorption
Calcium Chloride Injection is 100% bioavailable following intravenous injection.
Distribution
Calcium in the body is distributed mainly in skeleton (99%) and 1% is distributed within the extracellular fluids and soft tissues. About 50% of total serum calcium is in the ionized form and represents the biologically active part; 8% to 10% serum calcium is bound to organic and inorganic acid, respectively; and approximately 40% is protein-bound (primarily to albumin).
Elimination
Metabolism
Calcium itself does not undergo direct metabolism.
Excretion
Calcium is excreted by the kidney through a combination of glomerular filtration and tubular reabsorption. A significant increase in urinary excretion of calcium was observed during and after intravenous infusion of calcium chloride.
Specific Populations
The effect of age, sex, race, ethnicity, renal or hepatic impairment on the pharmacokinetics of calcium have not been evaluated in clinical studies.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
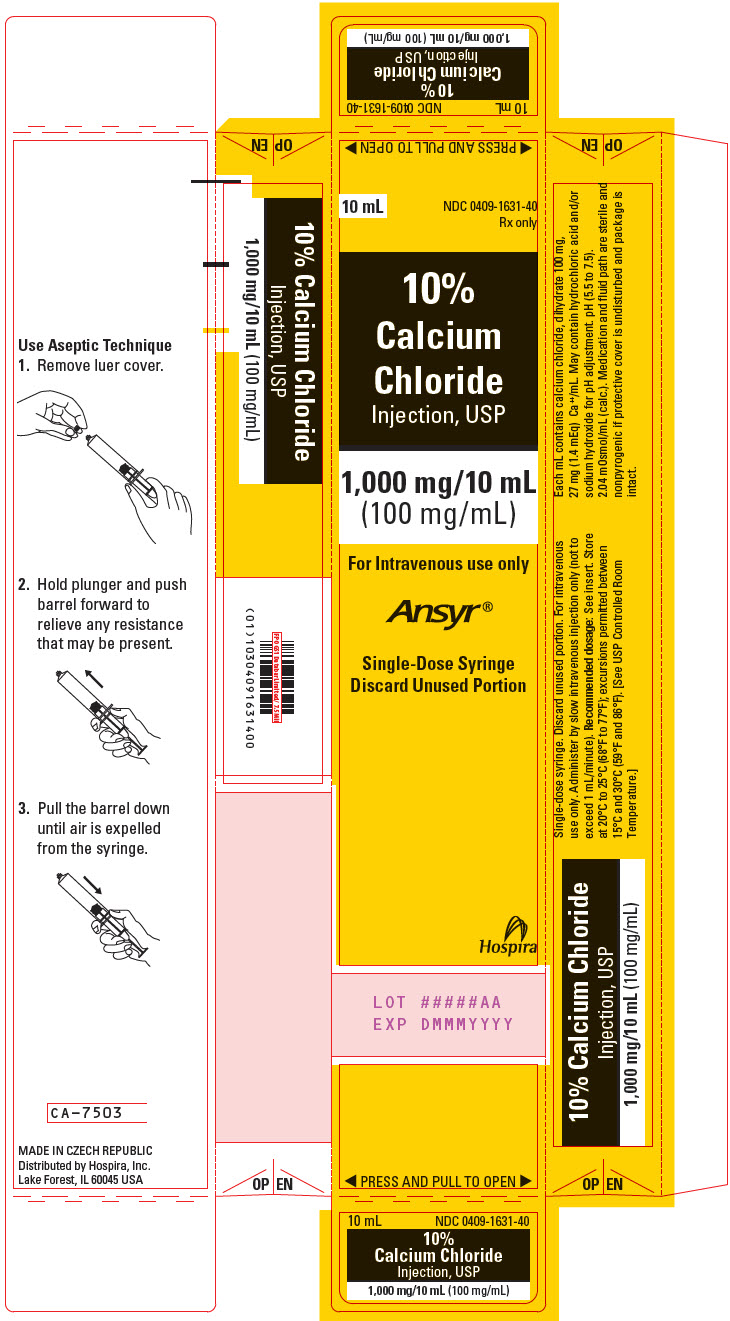
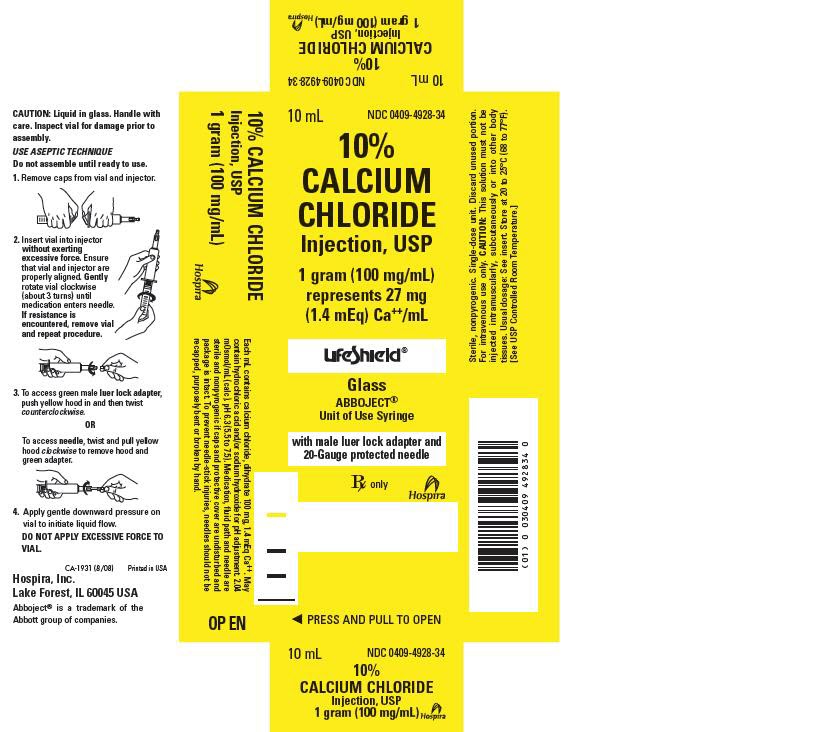
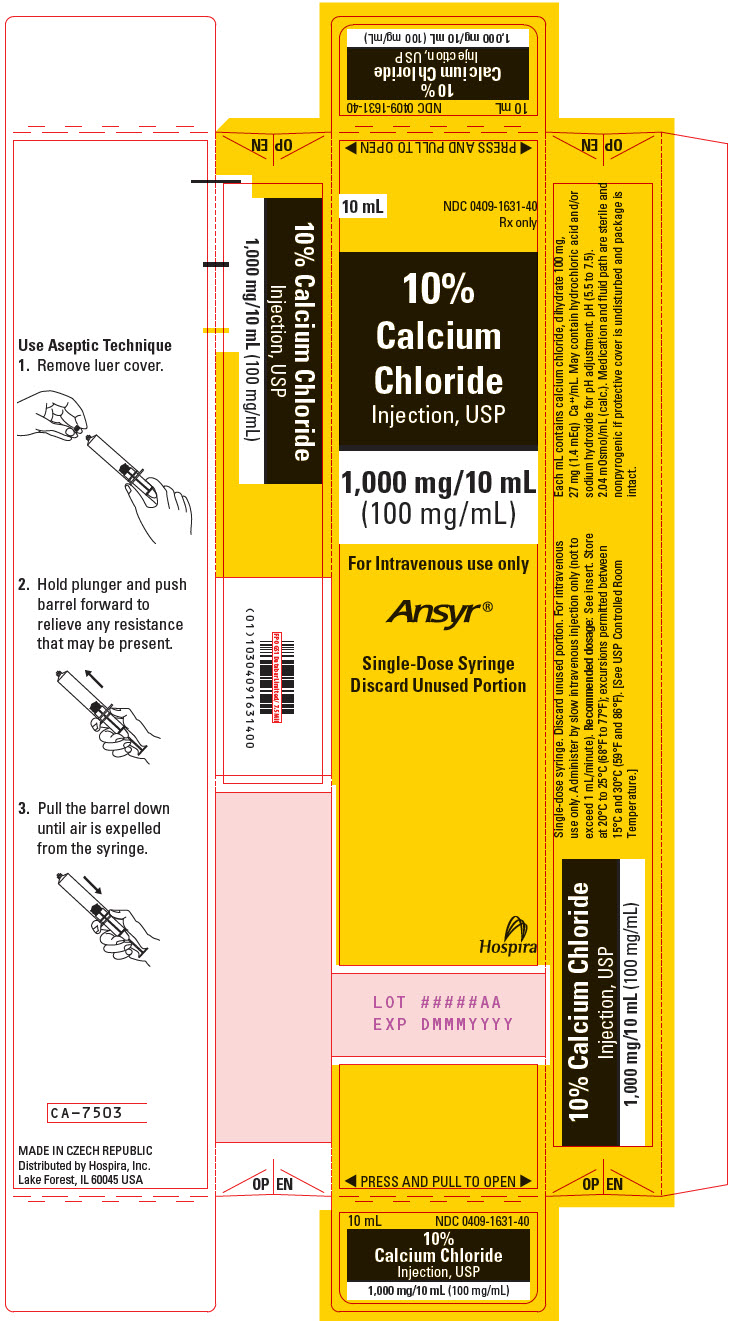
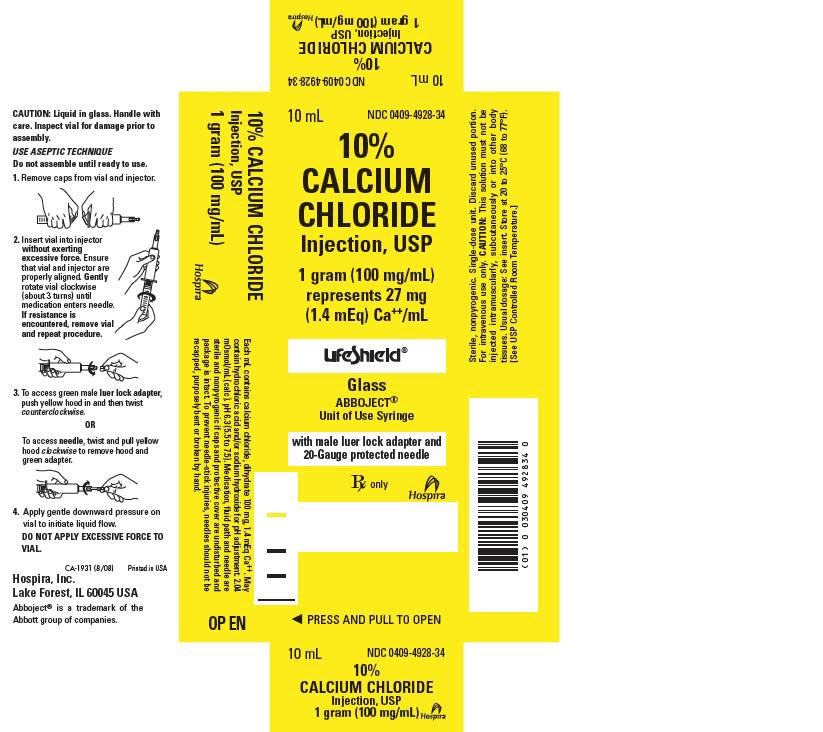
Calcium Chloride Injection, USP (clear solution) is supplied in single-dose syringes as follows:
Unit of Sale and Product Description
Strength
NDC
Bundle of 10
10 mL Ansyr® Plastic Syringe
10% (1,000 mg/10 mL)
(100 mg/mL)
0409-1631-10
Bundle of 10
10 mL LifeShield® Abboject® Glass Syringe with Luer Lock Adapter and protected needle
10% (1,000 mg/10 mL)
(100 mg/mL)
0409-4928-34
The 100 mg/mL concentration represents 27 mg or 1.4 mEq of elemental calcium per mL of solution.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). [See USP Controlled Room Temperature.]
-
17 PATIENT COUNSELING INFORMATION
Inform patients or caregivers of the following risks of Calcium Chloride Injection:
Arrhythmias with Concomitant Digoxin Use
Arrhythmias may occur if Calcium Chloride Injection and Digoxin are administered together [see Warnings and Precautions (5.3)].
Tissue Necrosis and Calcinosis
Administration of Calcium Chloride Injection may result in calcinosis cutis including tissue necrosis, ulceration, and secondary infection. [see Warnings and Precautions (5.4)].
Aluminum Toxicity
Calcium Chloride Injection contains aluminum that may be toxic [see Warnings and Precautions (5.5)].
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Abboject® is a trademark of Abbott Laboratories
LifeShield® is the trademark of ICU Medical, Inc. and is used under license.
LAB-1022-3.0
-
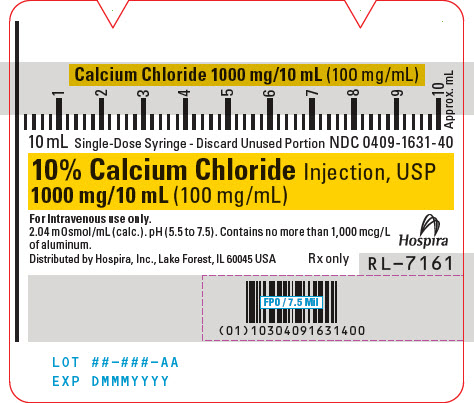
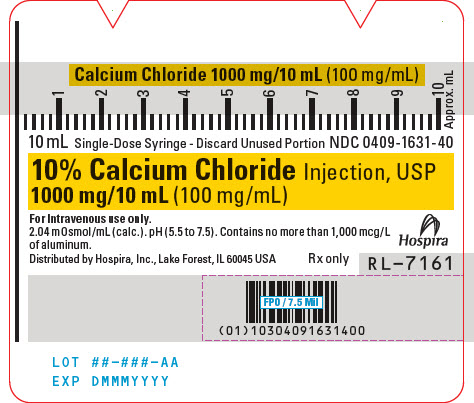
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
10 mL Single-Dose Syringe - Discard Unused Portion NDC 0409-1631-40
10% Calcium Chloride Injection, USP
1000 mg/10 mL (100 mg/mL)For Intravenous use only.
2.04 mOsmol/mL (calc.). pH (5.5 to 7.5). Contains no more than 1,000 mcg/L
of aluminum.Hospira
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Rx onlyRL-7161
- PRINCIPAL DISPLAY PANEL - 10 mL Syringe Carton
-
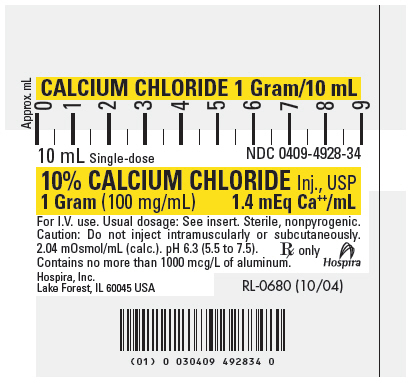
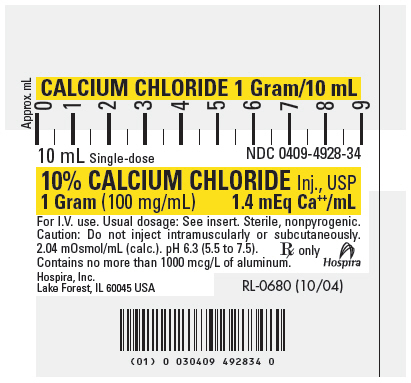
PRINICIPAL DISPLAY PANEL – 10mL Syringe Label
CALCIUM CHLORIDE 1 Gram/10 mL
10 mL Single-dose
NDC 0409-4928-3410% CALCIUM CHLORIDE Inj., USP
1 Gram (100 mg/mL)
1.4 mEq Ca++/mLFor I.V. use. Usual dosage: See insert. Sterile, nonpyrogenic.
Caution: Do not inject intramuscularly or subcutaneously.2.04 mOsmol/mL (calc.). pH 6.3 (5.5 to 7.5).
Contains no more than 1000 mcg/L of aluminum.Rx only
Hospira
Hospira, Inc.
Lake Forest, IL 60045 USARL-0680 (10/04)
- PRINCIPAL DISPAY PANEL – 10mL Syringe Carton
-
INGREDIENTS AND APPEARANCE
CALCIUM CHLORIDE
calcium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-1631 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-1631-10 10 in 1 CONTAINER 09/14/2005 1 NDC:0409-1631-40 1 in 1 CARTON 1 10 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021117 09/14/2005 CALCIUM CHLORIDE
calcium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0409-4928 Route of Administration INTRAVENOUS, INTRAVENTRICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0409-4928-34 10 in 1 CONTAINER 01/24/2006 1 1 in 1 CARTON 1 10 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 01/24/2006 Labeler - Hospira, Inc. (141588017) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(0409-1631, 0409-4928) , MANUFACTURE(0409-1631, 0409-4928) , PACK(0409-1631, 0409-4928) , LABEL(0409-1631, 0409-4928) Establishment Name Address ID/FEI Business Operations Hospira Worldwide, LLC 963711309 ANALYSIS(0409-1631)