6800 PORTEX CENTRAL LINE DRESSING- regional anesthesia kit
Smiths Medical ASD, Inc.
----------
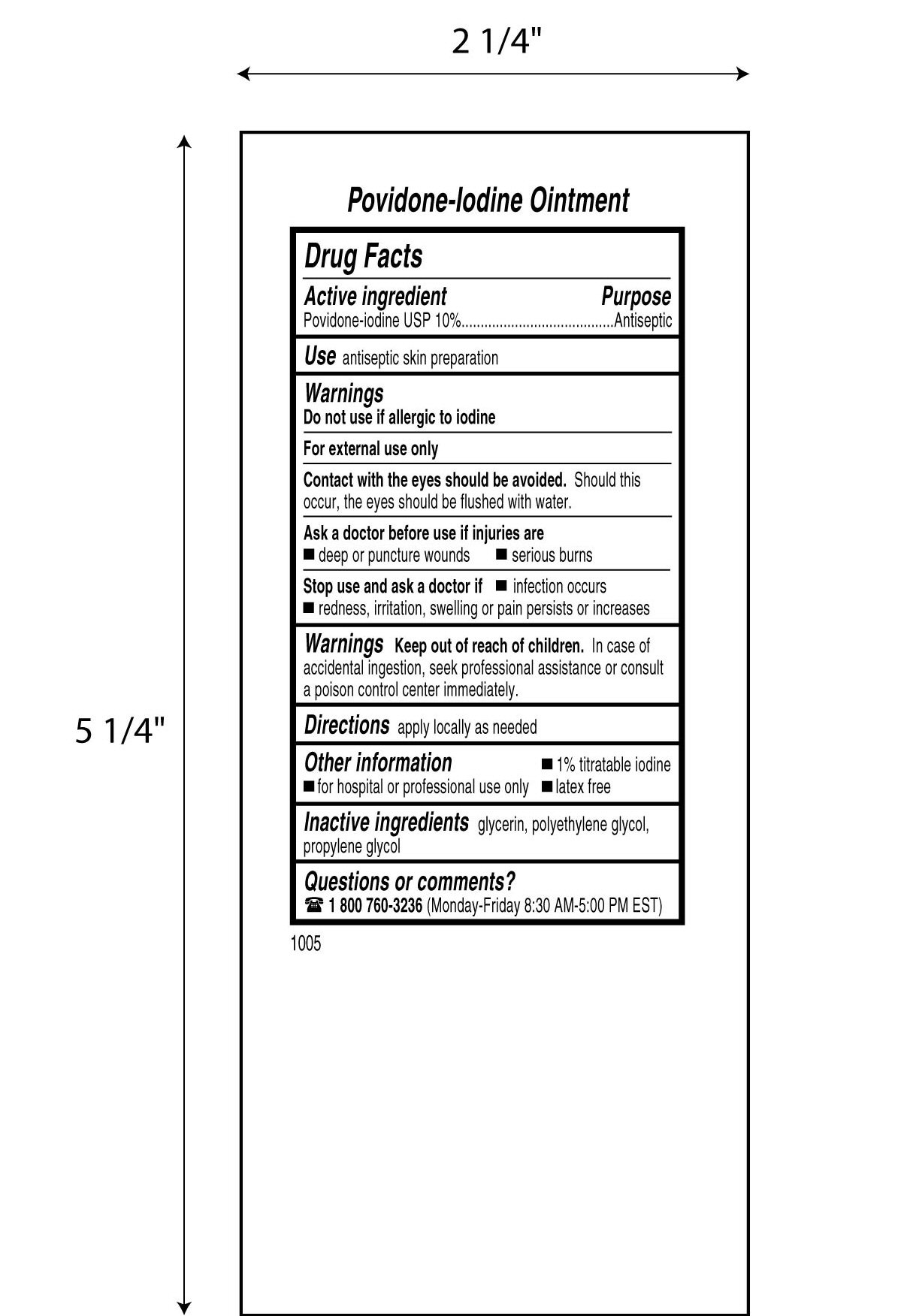
Drug Facts
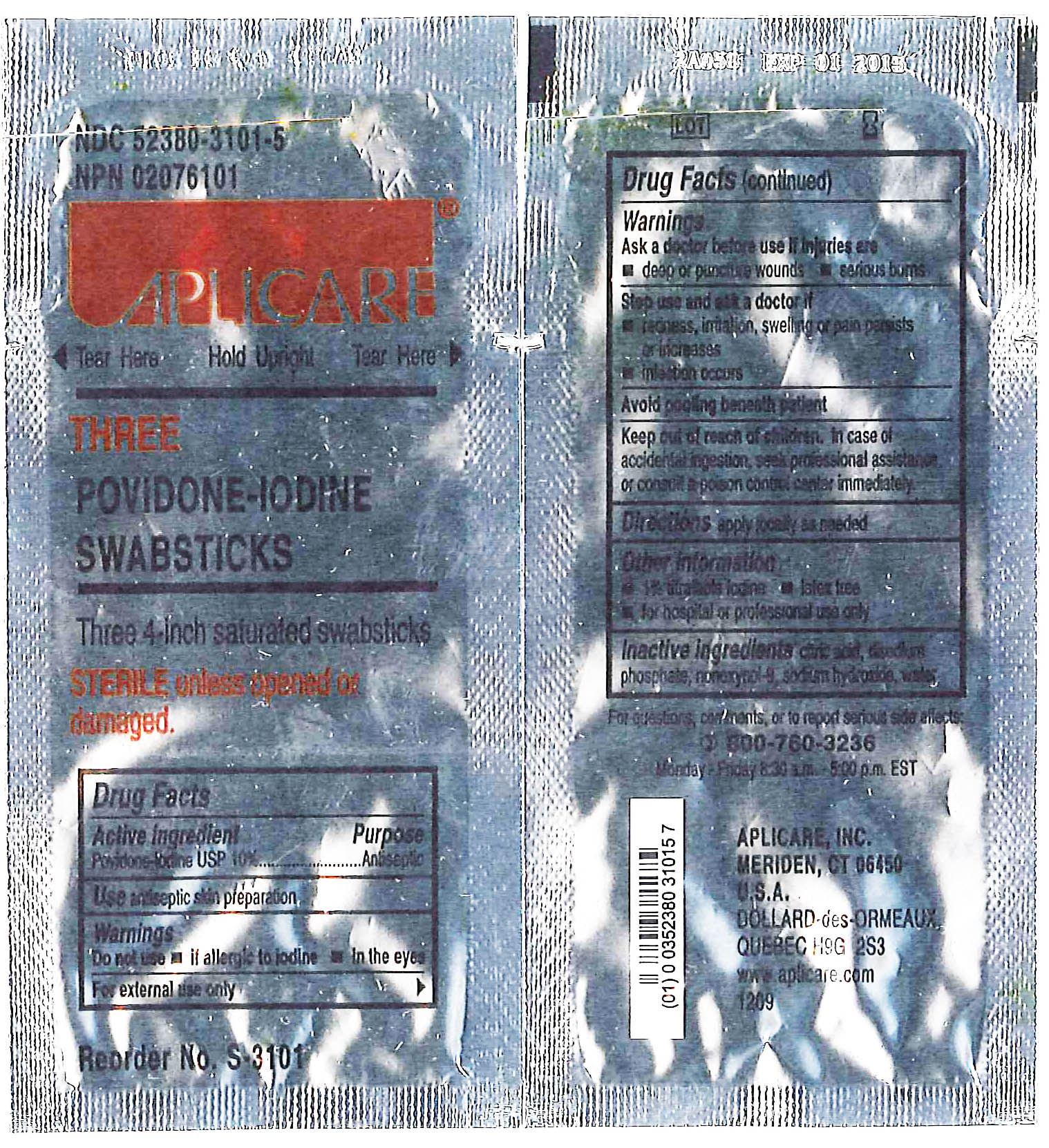
APLICARE THREE POVIDONE-IODINE SWABSTICKS
[Aplicare, Inc.]
Three 4-inch saturated swabsticks
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children.In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
| 6800 PORTEX CENTRAL LINE DRESSING
regional anesthesia kit kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Smiths Medical ASD, Inc. (137835299) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smiths Medical ASD, Inc. | 137835299 | relabel, manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare, Inc. | 081054904 | manufacture | |
Revised: 1/2020
Document Id: 45bab771-624c-41ed-be7f-26bc37727ec7
Set id: c4c4b8c4-6102-4001-938b-b27af190117c
Version: 2
Effective Time: 20200103
Smiths Medical ASD, Inc.