ANTIFUNGAL FOOT- clotrimazole cream
HyVee Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
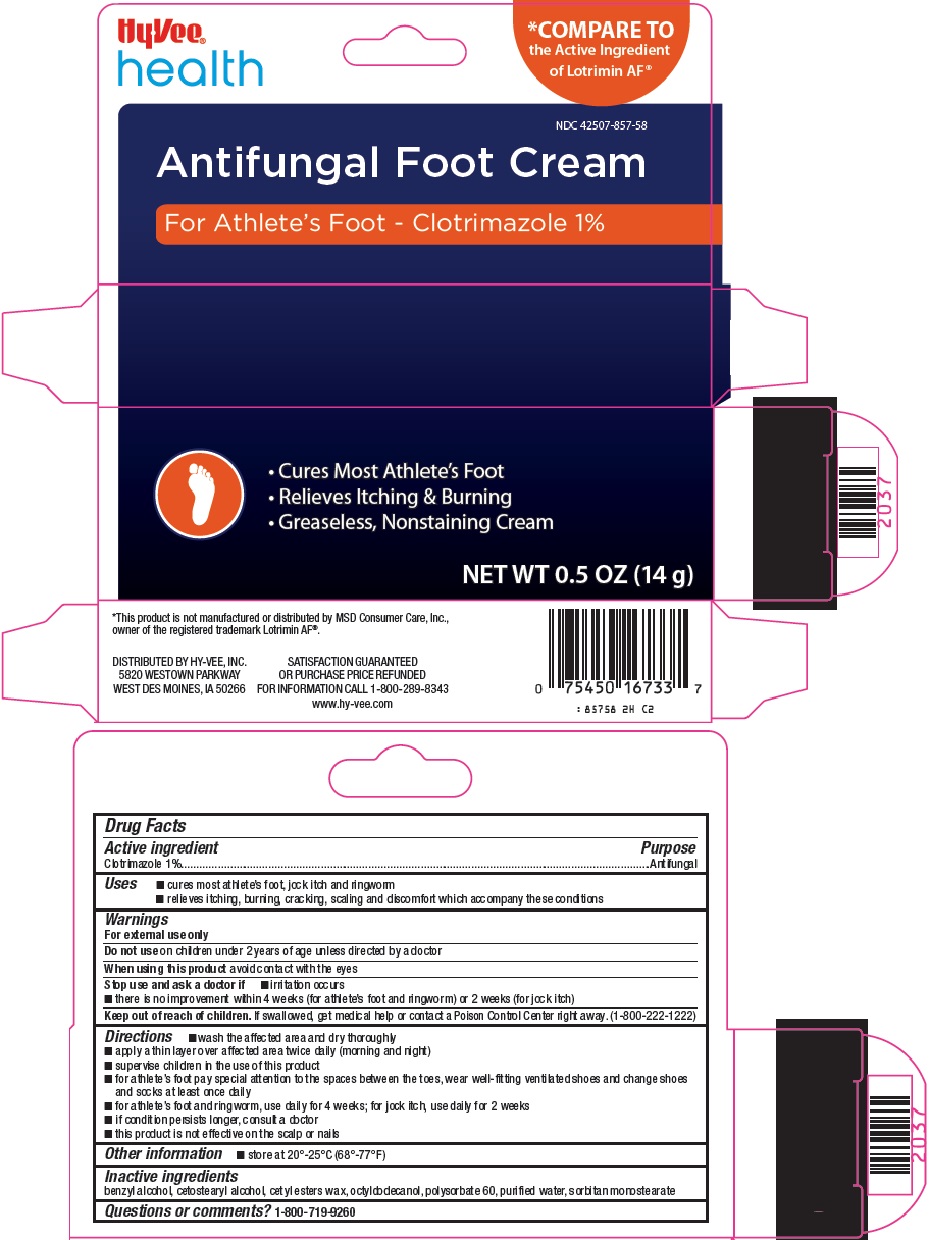
Hy-Vee, Inc. Antifungal Foot Cream Drug Facts
Uses
- •
- cures most athlete’s foot, jock itch and ringworm
- •
- relieves itching, burning, cracking, scaling and discomfort which accompany these conditions
Warnings
For external use only
Directions
- •
- wash the affected area and dry thoroughly
- •
- apply a thin layer over affected area twice daily (morning and night)
- •
- supervise children in the use of this product
- •
- for athlete’s foot pay special attention to the spaces between the toes, wear well-fitting ventilated shoes and change shoes and socks at least once daily
- •
- for athlete’s foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- •
- if condition persists longer, consult a doctor
- •
- this product is not effective on the scalp or nails
| ANTIFUNGAL FOOT
clotrimazole cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - HyVee Inc (006925671) |
Revised: 7/2019
Document Id: 2e917e8c-6c06-43b3-a465-64fb7f334a82
Set id: c4bcd489-fbdf-4023-8fde-56ebe8dc1b59
Version: 3
Effective Time: 20190725
HyVee Inc