Label: GOOD CARE POVIDONE IODINE- povidone-iodine swab
- NDC Code(s): 50685-001-01, 50685-001-03
- Packager: Dalian Goodwood Medical Care Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
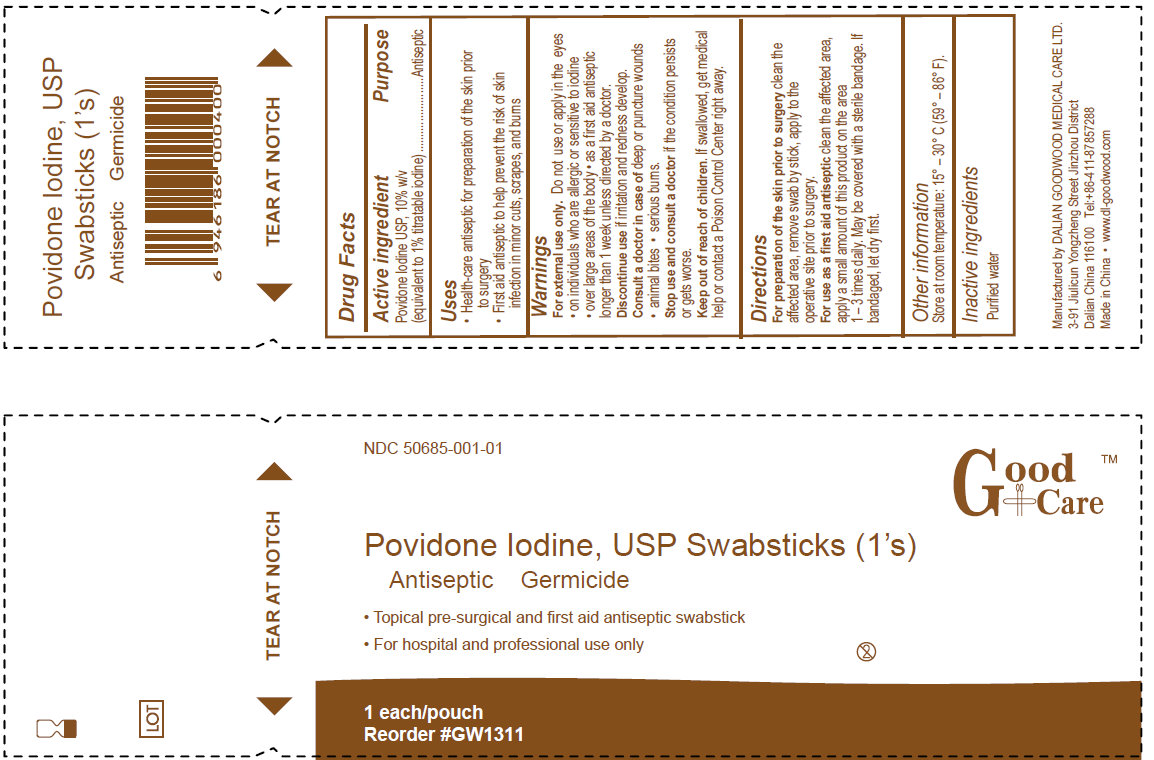
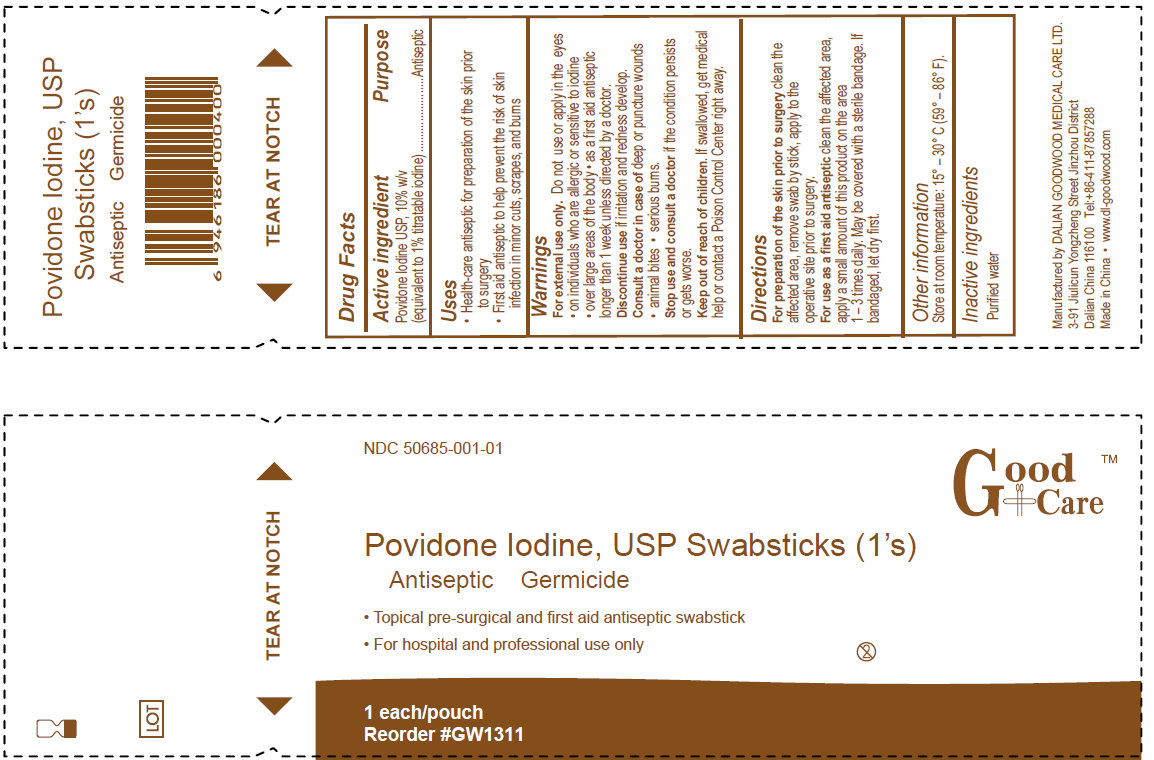
For preparation of the skin prior to surgery clean the affected area, remove swab by the stick, apply to the operative site prior to surgery.
For use as a first aid antiseptic clean the affected area, apply a small amount of this product on the area 1-3 times daily. May be covered with a sterile bandage. If bandaged, let dry first.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOOD CARE POVIDONE IODINE
povidone-iodine swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50685-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50685-001-01 2 mL in 1 POUCH; Type 0: Not a Combination Product 10/01/2010 04/30/2026 2 NDC:50685-001-03 5.5 mL in 1 POUCH; Type 0: Not a Combination Product 10/01/2010 04/30/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 10/01/2010 04/30/2026 Labeler - Dalian Goodwood Medical Care Ltd. (529575699) Registrant - Dalian Goodwood Medical Care Ltd. (529575699) Establishment Name Address ID/FEI Business Operations Dalian Goodwood Medical Care Ltd. 529575699 manufacture(50685-001)