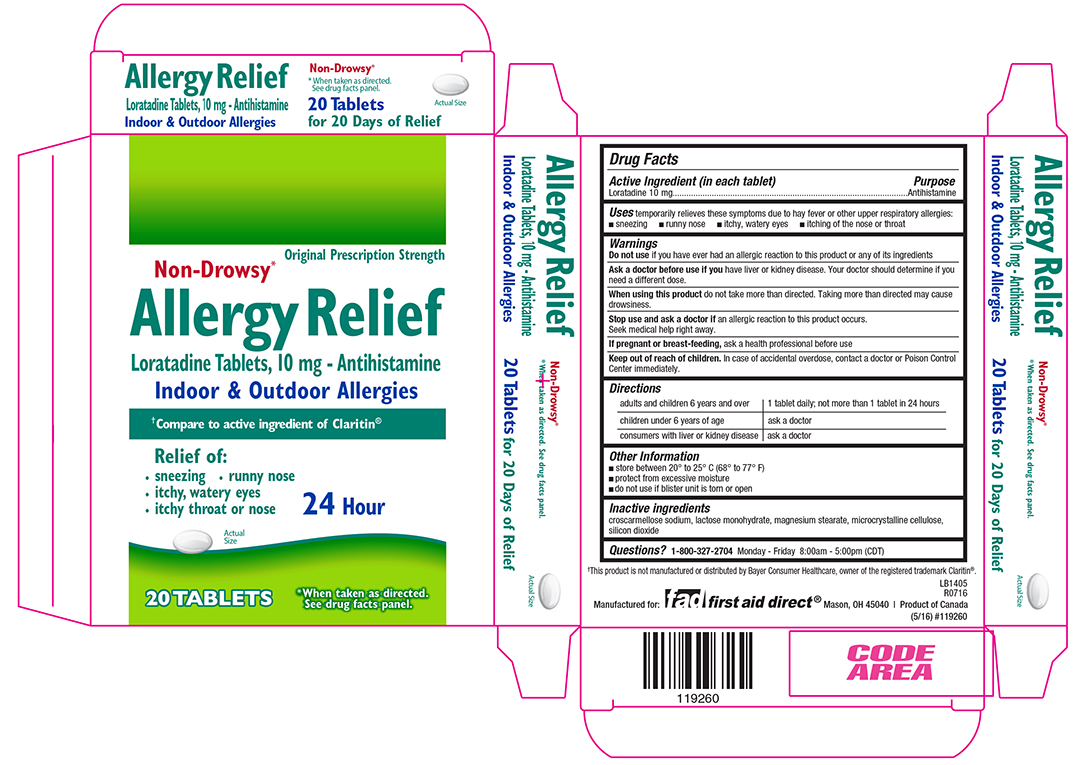
ALLERGY RELIEF- loratadine tablet
First Aid Direct
----------
Drug Facts
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- sneezing
- runny nose
- itchy, watery eyes
- itching of the nose or throat
Warnings
Ask doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
Keep out of reach of children.
In case of accidental overdose, contact a doctor or Poison Control Center immediately.
Directions
Adults and children 6 years and over: 1 tablet daily: not more than 1 tablet in 24 hours
Children under 6 years of age: ask a doctor
Consumers with liver or kidney disease: ask a doctor
| ALLERGY RELIEF
loratadine tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - First Aid Direct (962283128) |
Revised: 12/2017
Document Id: 6052d6e5-94bd-4844-8436-7d71e6d3eeaa
Set id: c45d6879-69a3-403d-8f02-a21af6027d2d
Version: 5
Effective Time: 20171222
First Aid Direct