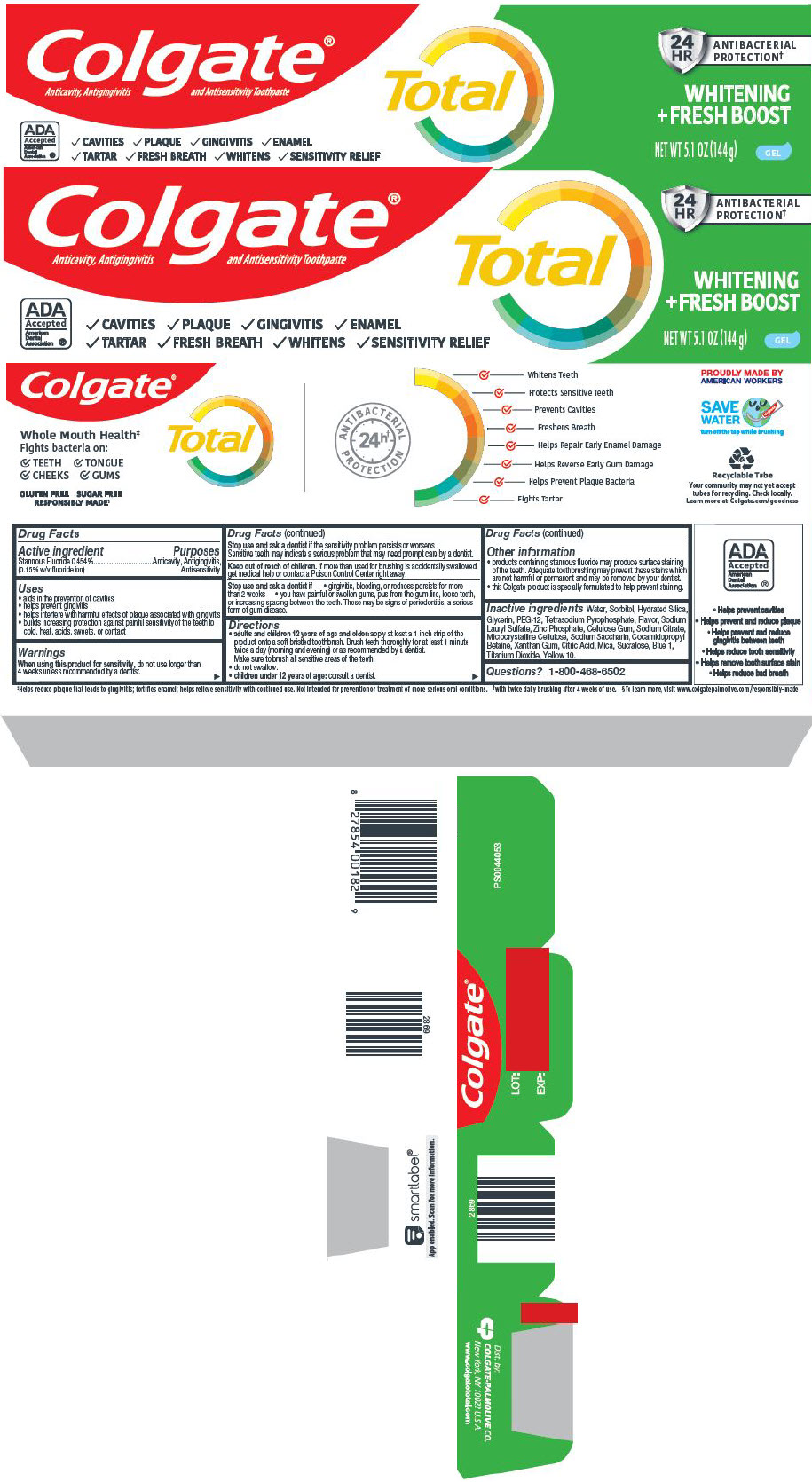
Label: COLGATE TOTAL WHITENING FRESH BOOST- stannous fluoride gel, dentifrice
- NDC Code(s): 35000-179-48, 35000-179-65, 35000-179-66
- Packager: Colgate-Palmolive Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purposes
- Uses
-
Warnings
When using this product for sensitivity, do not use longer than 4 weeks unless recommended by a dentist.
Stop use and ask a dentist if the sensitivity problem persists or worsens.
Sensitive teeth may indicate a serious problem that may need prompt care by a dentist.
-
Directions
-
adults and children 12 years of age and older: apply at least a 1-inch strip of the product onto a soft bristled toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist.
Make sure to brush all sensitive areas of the teeth. - do not swallow.
- children under 12 years of age: consult a dentist.
-
adults and children 12 years of age and older: apply at least a 1-inch strip of the product onto a soft bristled toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist.
- Other information
-
Inactive ingredients
Water, Sorbitol, Hydrated Silica, Glycerin, PEG-12, Tetrasodium Pyrophosphate, Flavor, Sodium Lauryl Sulfate, Zinc Phosphate, Cellulose Gum, Sodium Citrate, Microcrystalline Cellulose, Sodium Saccharin, Cocamidopropyl Betaine, Xanthan Gum, Citric Acid, Mica, Sucralose, Blue 1, Titanium Dioxide, Yellow 10.
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 144 g Tube Carton
-
INGREDIENTS AND APPEARANCE
COLGATE TOTAL WHITENING FRESH BOOST
stannous fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:35000-179 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.1 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) HYDRATED SILICA (UNII: Y6O7T4G8P9) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 600 (UNII: NL4J9F21N9) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ZINC PHOSPHATE (UNII: 1E2MCT2M62) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SACCHARIN SODIUM (UNII: SB8ZUX40TY) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) XANTHAN GUM (UNII: TTV12P4NEE) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SUCRALOSE (UNII: 96K6UQ3ZD4) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color GREEN Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:35000-179-48 1 in 1 CARTON 12/01/2021 12/30/2024 1 136 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:35000-179-66 1 in 1 CARTON 12/27/2021 2 144 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:35000-179-65 2 in 1 CARTON 11/26/2021 3 144 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M021 11/26/2021 Labeler - Colgate-Palmolive Company (001344381)