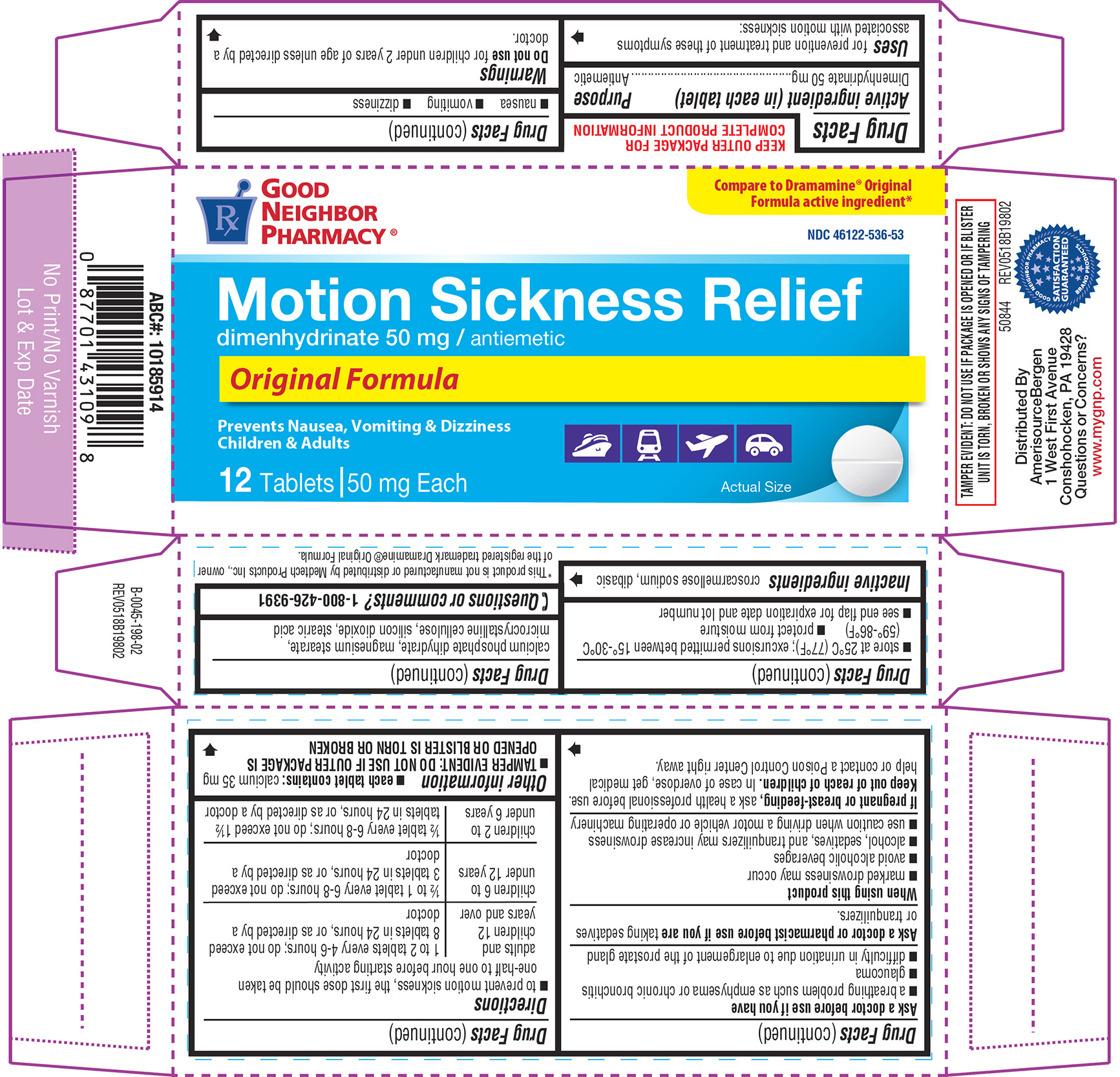
Label: MOTION SICKNESS RELIEF- dimenhydrinate tablet
- NDC Code(s): 46122-536-53
- Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 12, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
-
Directions
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
adults and children 12 years and over 1 to 2 tablets every 4-6 hours; do not exceed 8 tablets in 24 hours, or as directed by a doctor
children 6 to under 12 years
½ to 1 tablet every 6-8 hours; do not exceed 3 tablets in 24 hours, or as directed by a doctor
children 2 to under 6 years
½ tablet every 6-8 hours; do not exceed 1½ tablets in 24 hours, or as directed by a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
GOOD
NEIGHBOR
PHARMACY®NDC 46122-536-53
Compare to Dramamine® Original
Formula active ingredient*Motion Sickness Relief
dimenhydrinate 50 mg / antiemetic
Original Formula
Prevents Nausea, Vomiting & Dizziness
Children & Adults12 Tablets | 50 mg Each
Actual Size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING50844 REV0518B19802
Distributed By
AmerisourceBergen
1 West First Avenue
Conshohocken, PA 19428
Questions or Concerns?
www.mygnp.com
GNP 44-198
-
INGREDIENTS AND APPEARANCE
MOTION SICKNESS RELIEF
dimenhydrinate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-536 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (DIPHENHYDRAMINE - UNII:8GTS82S83M, 8-CHLOROTHEOPHYLLINE - UNII:GE2UA340FM) DIMENHYDRINATE 50 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code 44;198 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-536-53 2 in 1 CARTON 12/01/1992 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 12/01/1992 Labeler - Amerisource Bergen (007914906) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(46122-536) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(46122-536) , pack(46122-536) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(46122-536)