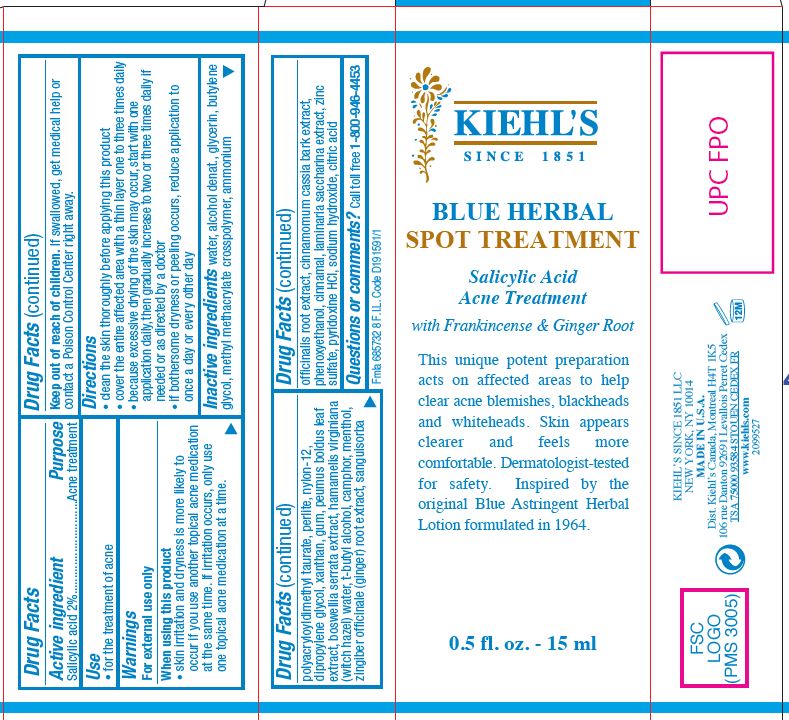
BLUE HERBAL SPOT TREATMENT SALICYLIC ACID ACNE TREATMENT- salicylic acid gel
L'Oreal USA Products Inc
----------
Drug Facts
When using this products
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
water, alcohol denat., glycerin, butylene glycol, methyl methacrylate crosspolymer, ammonium polyacryloyldimethyl taurate, perlite, nylon-12, dipropylene glycol, xanthan gum, peumus boldus leaf extract, boswellia serrata extract, hamamelis virginiana (witch hazel) water, t-butyl alcohol, camphor, menthol, zingiber officinale (ginger) root extract, sanuisorba officinalis root extract, cinnamomum cassia bark extract, phenoxyethanol, cinnamal, laminaria saccharina extract, zinc sulfate, pyridoxine HCl, sodium hydroxide, citric acid
| BLUE HERBAL SPOT TREATMENT SALICYLIC ACID ACNE TREATMENT
salicylic acid gel |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - L'Oreal USA Products Inc (002136794) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cosmetic Essence, LLC | 032565959 | manufacture(49967-602) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Paklab | 177711082 | pack(49967-602) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| L'OREAL USA, INC. | 185931458 | analysis(49967-602) | |
Revised: 1/2024
Document Id: 80b8bfe4-452e-4c67-bbc7-3fb10179b915
Set id: c3b008ae-615b-4717-a82f-694dd14fe849
Version: 15
Effective Time: 20240108
L'Oreal USA Products Inc