LISTERINE NATURALS ANTICAVITY - HERBAL MINT- sodium fluoride mouthwash
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
LISTERINE® naturals™ ANTICAVITY MOUTHWASH HERBAL MINT
Directions
- Adults and children 12 years of age and older:
- use twice daily after brushing your teeth with a toothpaste
- vigorously swish 10 ml (2 teaspoonfuls) of rinse between your teeth for 1 minute and then spit out
- do not swallow the rinse
- do not eat or drink for 30 minutes after rinsing
- supervise children as necessary until capable of using without supervision
- Children under 12 years of age: consult a dentist or doctor
Inactive ingredients
water, sorbitol solution, alcohol (21.6%), poloxamer 407, flavor, eucalyptol, methyl salicylate, thymol, stevia rebaudiana leaf/stem extract, phosphoric acid, menthol, disodium phosphate
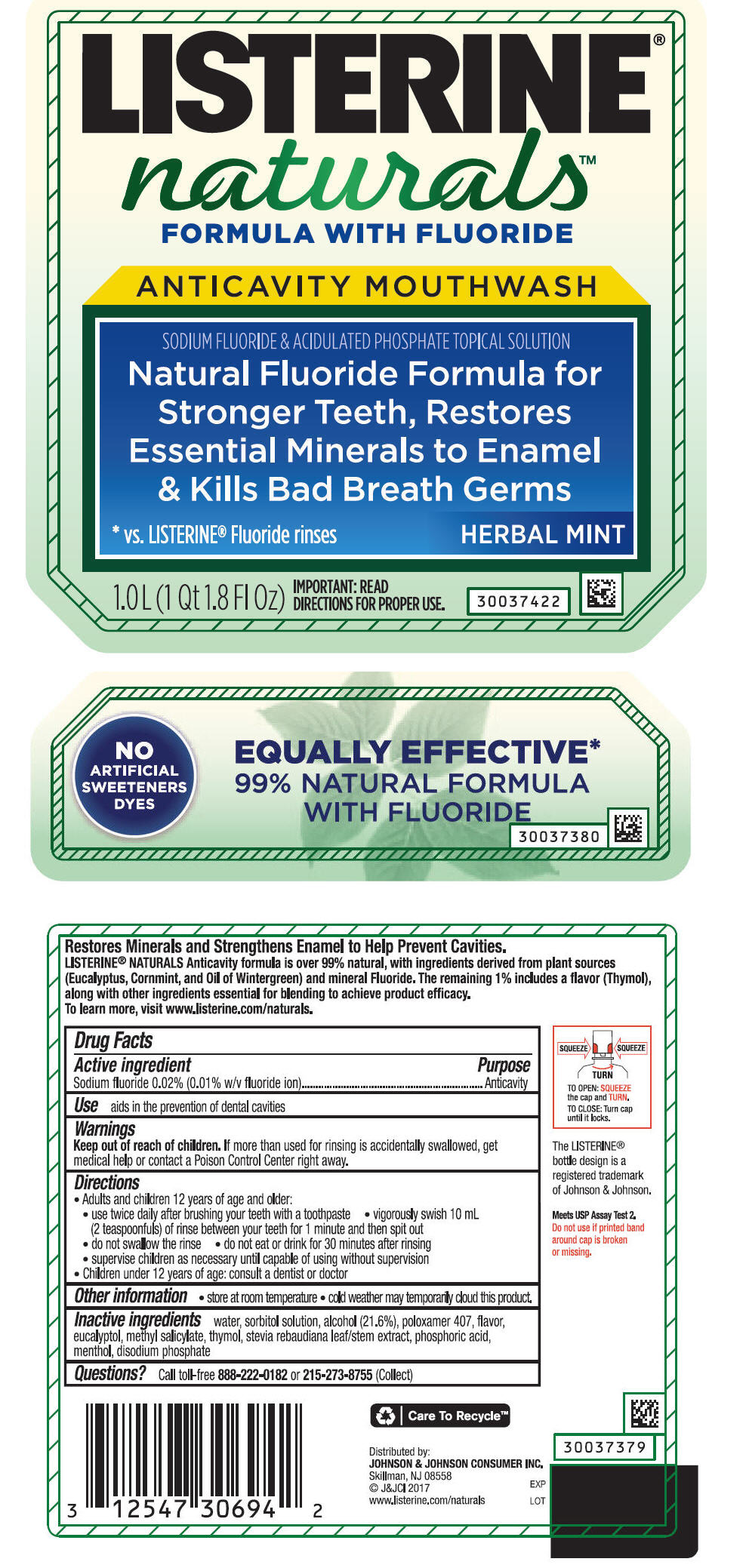
PRINCIPAL DISPLAY PANEL - 1.0 L Bottle Label
LISTERINE®
naturals™
FORMULA WITH FLUORIDE
ANTICAVITY MOUTHWASH
SODIUM FLUORIDE & ACIDULATED PHOSPHATE TOPICAL SOLUTION
Natural Fluoride Formula for
Stronger Teeth, Restores
Essential Minerals to Enamel
& Kills Bad Breath Germs
| *vs. LISTERINE® Fluoride rinses | HERBAL MINT |
1.0L (1 Qt 1.8 Fl OZ)
IMPORTANT: READ
DIRECTIONS FOR PROPER USE.
| LISTERINE NATURALS ANTICAVITY - HERBAL MINT
sodium fluoride mouthwash |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |