Label: HEMATOGEN- ferrous fumarate, ascorbic acid, intrinsic factor , cyanocobalamin capsule
- NDC Code(s): 63044-631-19
- Packager: Nnodum Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Ferrous fumarate (66 mg elemental iron) USP................... 200 mg
Ascorbic acid USP.................................................................. 250 mg
Desiccated stomach powder................................................... 100 mg
(Contains: Intrinsic factor),
Cyanocobalamin USP...............................................................10 mcg
DISCUSSION: The amount of elemental iron and the absorption of the iron components of commercial iron preparations vary widely. It is further established that certain "accessory components" may be included to enhance absorption and utilization of iron. Hematogen™ Capsules are formulated to provide the essential factors for a complete, versatile hematinic.
-
ACTIONS/CLINICAL PHARMACOLOGY
HIGH ELEMENTAL IRON CONTENT: Ferrous fumarate, used in Hematogen™ Capsules, is an organic iron complex which has the highest elemental iron content of any hematinic salt-33%. This compares with 20% for ferrous sulfate (heptahydrate) and 13% for ferrous gluconate. Hematogen™ contains 66 mg of elemental iron.
MORE COMPLETE ABSORPTION: It has been repeatedly shown that ascorbic acid, when given in sufficient amounts, can increase the absorption of ferrous iron from the gastrointestinal tract. The absorption promoting effect is mainly due to the reducing action of ascorbic acid within the gastro intestinal lumen, which helps to prevent or delay the formation of insoluble or less dissociated ferric compounds.
Iron absorption has been shown to increase sharply with increasing amounts of ascorbic acid, showing a gain in absorption of approximately 40% at 250 mg. Above 250 mg, the gain becomes insignificant, with an additional gain of only approximately 8% at 500 mg. Each Hematogen™ Capsule contains 250 mg of ascorbic acid, believed to be the optimal amount.
PROMOTES MOVEMENT OF PLASMA IRON: Ascorbic acid also plays an important role in the movement of plasma iron to storage depots in the tissues . The action, which leads to the transport of plasma iron to ferritin, presumably involves its reducing effect; converting transferrin iron from 5 the ferric to the ferrous state. There is also evidence that ascorbic acid improves iron utilization,
presumably as a further result of its reducing action , and some evidence that it may have a direct effect upon erythropoiesis. Ascorbic acid is further alleged to enhance the conversion of folic acid to a more physiologically active form, folinic acid. which would make it even more important in the 11 treatment of anemia since it would aid in the utilization of dietary folic acid.
EXCELLENT ORAL TOLERATION: Ferrous fumarate is used in Hematogen™ Capsules because it is less likely to cause the gastric disturbances so often associated with oral iron therapy. Ferrous fumarate has a low ionization constant and high solubility in the entire pH range of the gastrointestinal tract.
It does not precipitate proteins or have the astringency of more ionizable forms of iron, and does not interfere with proteolytic or diastatic activities of the digestive system. Because of excellent oral toleration, Hematogen™ Capsules can usually be administered between meals when iron absorption is maximal.
FACILITATES ABSORPTION OF VITAMIN B12 : It is now known that "Intrinsic Factor" is essential for the adequate alimentary absorption of vitamin B. The inclusion of desiccated stomach powder 12 with oral vitamin B will furnish sufficient intrinsic factor to assure absorption of the vitamin 12
only
TOXICITY: Ferrous fumarate was found to be the least toxic of three popular oral iron salts, with an oral LD50 of 630 mg/kg. In the same report, the LD50 of ferrous gluconate was reported to be 320 mg/kg and ferrous sulfate 230 mg/kg.
- INDICATIONS:
-
CONTRAINDICATIONS:
Hemochromatosis and hemosiderosis are contraindications to iron therapy.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. - ADVERSE REACTIONS:
- DOSAGE AND ADMINISTRATION:
-
HOW SUPPLIED:
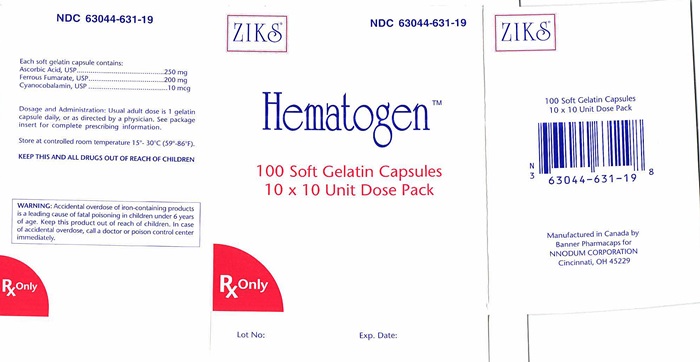
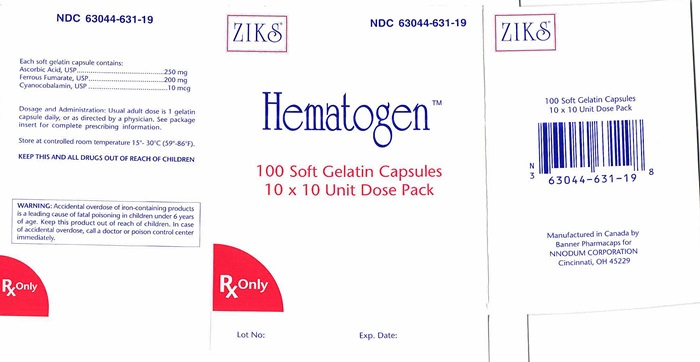
Each Red soft gelatin capsule is imprinted with "Ziks631" on one side. NDC 63044-0631-19 10 x 10, unit dose packs, in packs of 100's.
Store at controlled between temperature 15°- 30°C (59°- 86°F). Protect from direct / excessive heat 40°C (104°F). Avoid freezing.
1 BIBLIOGRAPHY: Berk, M.S. and Novich, M.A.: 'Treatment of Iron Deficiency Anemia With Ferrous
2 Fumarate," Am. J. Obst. & Gynec., 203-206, 1962. Shapleigh, J.B., and Montgomery, A.; Am. Pract. &
3 Dig. Treat. 10-461, 1959. Brise, H. and Hallberg, L.: "Effect of Ascorbic Acid on Iron Absorption," Acta.
4 5 Med. Scand. 171 :376, 51 -58,1962. New Drugs, p. 309, AMA, Chicago, 1966. Mazur, A., Green, S. and Carleton, A.: "Mechanism of Plasma Iron Incorporation into Hepatic Ferritin," J. of Bio. Chem.
6 3:595-603, 1960. Greenberg, S.M., Tucker, A.E., Mathues, H. and J.D.: "Iron Absorption and
7 Metabolism, I. Interrelationship of Ascorbic Acid and Vitamin E," J. Nutrition 63:19-31, 1957. Moore, C. V., and Dubach, R.: "Observations on the Absorption of Iron From Foods Tagged with Radioiron,
8 "Trans. Assoc. Amer. Physic. 64:245, 1951. Steinkamp, R., Dubach, R. and Moore, C.Y. "Studies in
9 Iron Transportation and Metabolism," Arch. Int. Med. 95:181, 1955. Gorten, M.K. and Bradley, J.E.: "The Treatment of Nutritional Anemia in Infancy and Childhood with Oral Iron and Ascorbic
l0 Acid,"J. Pediatrics, 45: 1, 1954. Mazur, A.: "Role of Ascorbic Acid in the Incorporation of Plasma Iron
11 into Ferritin." An. N.Y. Acad. Sci. 92:223-229, 1961. COX, E. V. et al.: "The Anemia of Scurvy," Amer. J.
12 Med. 42:220-227, 1967. Berk, L. et al.: "Observations on the Etiologic Relationship of Achylia
13 Gastrica to Pernicious Anemia, X," N. Eng. J. Med. 239:911-913, 1948. Hall, B.E.: "Studies on the
14 Nature of the Intrinsic Factor of Castle," Brit. Med. J. 2:585-589,1950. Wallerstein, R.O. et al.: "Observations on the Etiologic Relationship of Achylia Gastrica to Pernicious Anemia, XV" J. Lab
15 & Clin. Med. 41 :363-375, 1953. Castle, W.B.: "Observations on the Etiologic Relationship of Achylia
16 Gastrica to Pernicious Anemia, 1," Am. J. Med. Sc.178:748-764, 1929.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMATOGEN
ferrous fumarate, ascorbic acid, intrinsic factor , cyanocobalamin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63044-631 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 66 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 250 mg INTRINSIC FACTOR (UNII: 70BT6OQT2Q) (INTRINSIC FACTOR - UNII:70BT6OQT2Q) INTRINSIC FACTOR 100 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 10 ug Product Characteristics Color RED Score no score Shape OVAL Size 4mm Flavor Imprint Code ziks;631 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63044-631-19 100 in 1 BOX 05/10/2007 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/10/2007 Labeler - Nnodum Pharmaceuticals (960457273) Registrant - Nnodum Pharmaceuticals (960457273) Establishment Name Address ID/FEI Business Operations Contract Pharmacal Corporation 057795122 manufacture(63044-631)