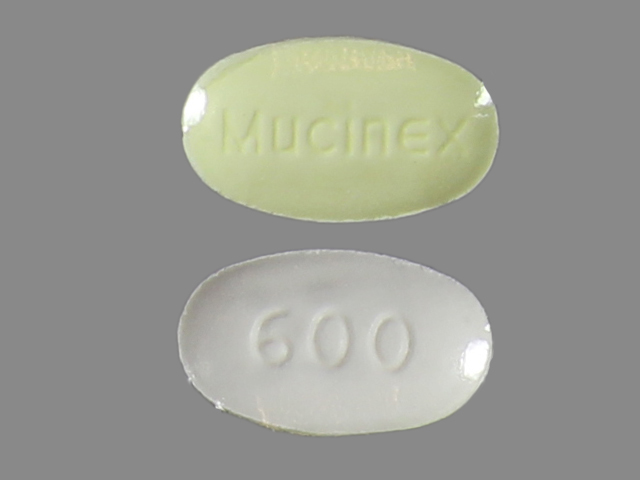
MUCINEX DM- guaifenesin and dextromethorphan hydrobromide tablet, extended release
American Health Packaging
----------
Mucinex
® DM
5500411/0122
Drug Facts
Active ingredients (in each extended-release bi-layer tablet)
Dextromethorphan HBr 30 mg
Guaifenesin 600 mg
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
Warnings
Do Not Use
- for children under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years and older: 1 or 2 extended-release tablets every 12 hours; not more than 4 extended-release tablets in 24 hours
- children under 12 years of age: do not use
Other information
- store at 20-25°C (68-77°F)
- FOR YOUR PROTECTION: Do not use if blister is torn or broken.
Inactive Ingredients
carbomer homopolymer type B; D&C yellow #10 aluminum lake; hypromellose, USP; magnesium stearate, NF; microcrystalline cellulose, NF; sodium starch glycolate, NF
Questions?
About the product, call RB Health (US) at 1-866-MUCINEX (1-866-682-4639)
You may also report side effects to this phone number.
For questions about the packaging and labeling, call American Health Packaging at 1-800 707-4621
The name MUCINEX ® and associated trademarks trademark items are used with permission by RB Health.
The drug product contained in this package is from NDC# 63824-056, RB Health (US).
Distributed by
American Health Packaging
2550 John Glenn Avenue, Suite A
Columbus, OH 43217
765121
5500411/0122
Package/Label Principal Display Panel – Carton – 600 mg/30 mg

NDC 60687- 651-21
Mucinex ® DM
600 mg guaifenesin & 30 mg dextromethorphan HBr
extended-release bi-layer tablets
EXPECTORANT & COUGH SUPPRESSANT
12 HOUR
Unique Bi-Layer Tablet
Immediate Release Layer
Extended Release Layer
30 Extended-Release Tablets (3 x 10)
Distributed by
American Health Packaging
2550 John Glenn Avenue, Suite A
Columbus, OH 43217
765121
5500411/0122
| MUCINEX DM
guaifenesin and dextromethorphan hydrobromide tablet, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - American Health Packaging (929561009) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| American Health Packaging | 929561009 | repack(60687-651) | |
