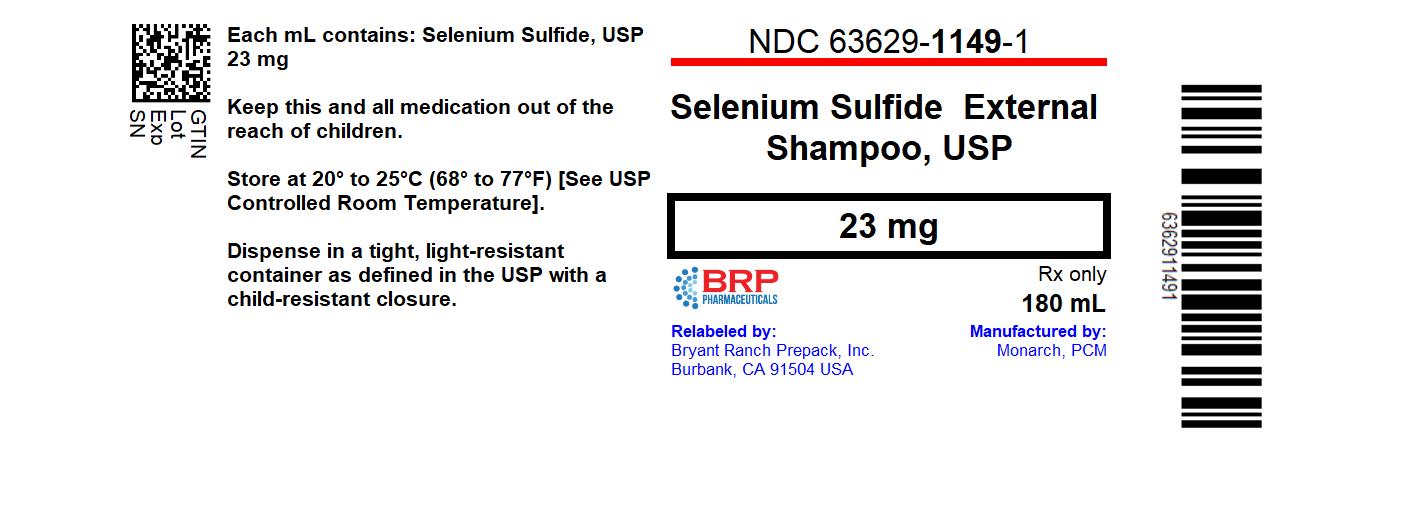
Label: SELENIUM SULFIDE shampoo
- NDC Code(s): 63629-1149-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 58657-479
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Selenium Sulfide 2.3% Shampoo
Rx Only
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
DESCRIPTION: Each mL contains 23 mg of selenium sulfide in a vehicle consisting of: alpha-tocopherol acetate, ammonium lauryl sulfate, citrus fragrance, cocamidopropyl betaine, EDTA, FD&C red No. 40, FD&C yellow No. 10, methyl paraben, panthenol, propyl paraben, purified water, pyrithione zinc, urea, xanthan gum.
- CLINICAL PHARMACOLOGY:
- INDICATIONS:
- CONTRAINDICATIONS:
- WARNINGS:
-
PRECAUTIONS:
General:
This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
Information for Patients:
Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. Avoid contact with eyes, lips and mucous membranes. If accidental contact occurs, rinse thoroughly with water. Do not use on broken skin or inflamed areas.
Carcinogenesis, Mutagenesis and Impairment of Fertility:
Dermal application of 25% and 50% solutions of 2.5% selenium sulfide lotion on mice over an 88-week period indicated no carcinogenic effects. Studies on reproduction and fertility also have not been performed.
Pregnancy:
Category C. Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
- ADVERSE REACTIONS:
-
OVERDOSAGE:
There are no documented reports of serious toxicity in humans resulting from acute ingestion of this product. However, acute toxicity studies in animals suggest that ingestion of large amounts could result in potential human toxicity. Evacuation of the stomach contents should be considered in cases of acute oral ingestion.
-
DOSAGE AND ADMINISTRATION:
Shake well before use.
For seborrheic dermatitis and dandruff: Wet skin and apply to areas to be cleansed. Massage gently into the skin working into a full lather. Rinse thoroughly and pat dry. Generally, two applications each week for two weeks will control symptoms. Subsequently, shampoo may be used less frequently or as directed by a physician. It should not be applied more frequently than necessary to maintain control.
For tinea versicolor: Wet skin and apply to areas to be cleansed. Massage gently into the skin working into a full lather. Allow product to remain on skin for ten minutes, then rinse thoroughly and pat dry. Repeat procedure once a day for seven days or as directed by a physician.
-
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (between 59°F to 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
NOTICE: Protect from freezing and excessive heat. The product may darken upon storage. Discoloration does not impair the efficacy or safety of the product. Keep container tightly closed.
- HOW SUPPLIED:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SELENIUM SULFIDE
selenium sulfide shampooProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-1149(NDC:58657-479) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM SULFIDE - UNII:Z69D9E381Q) SELENIUM SULFIDE 23 mg in 1 mL Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) EDETIC ACID (UNII: 9G34HU7RV0) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) METHYLPARABEN (UNII: A2I8C7HI9T) PANTHENOL (UNII: WV9CM0O67Z) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) PYRITHIONE ZINC (UNII: R953O2RHZ5) UREA (UNII: 8W8T17847W) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-1149-1 180 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/03/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/05/2018 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-1149) , RELABEL(63629-1149)