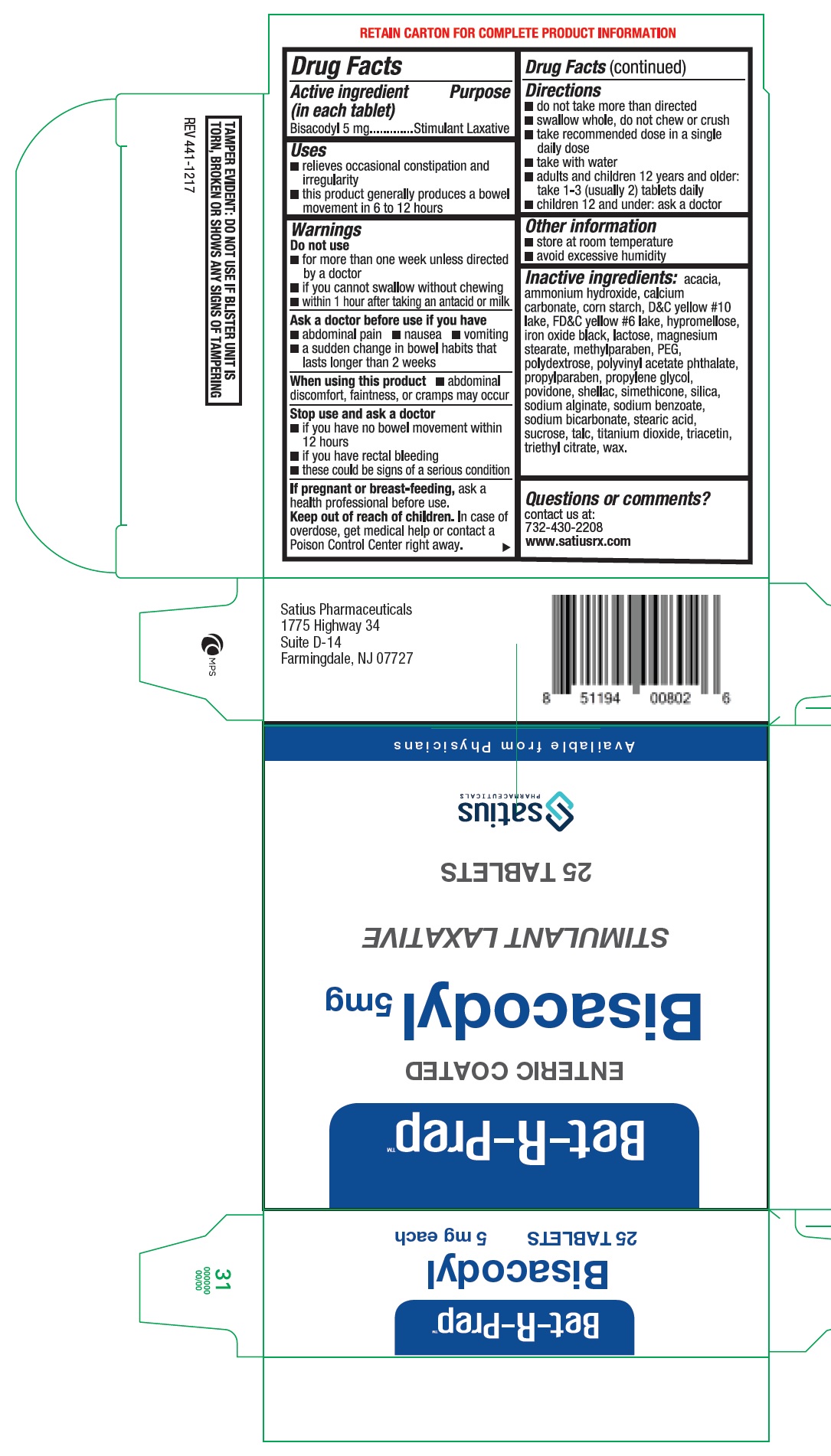
BET-R-PREP BISACODYL- bisacodyl tablet
Satius Pharmaceuticals, LLC
----------
BET-R-PREP Bisacodyl
Uses
- relieves occasional constipation and irregularity
- this product generally produces a bowel movement in 6 to 12 hours
Warnings
Do not use
- for more than one week unless directed by a doctor
- if you cannot swallow without chewing
- within 1 hour after taking an antacid or milk
Ask a doctor before use if you have
- abdominal pain
- nausea
- vomiting
- a sudden change in bowel habits that lasts longer than 2 weeks
Directions
- do not take more than directed
- swallow whole, do not chew or crush
- take recommended dose in a single daily dose
- take with water
- adults and children 12 years and older: take 1-3 (usually 2) tablets daily
- chidren 12 and under: ask a doctor
Inactive ingredients:
acacia, ammonium hydroxide, calcium carbonate, corn starch, D&C yellow #10 lake, FD&C yellow #6 lake, hypromellose, iron oxide black, lactose, magnesium stearate, methylparaben, PEG, polydextrose, polyvinyl acetate phthalate, propylparaben, propylene glycol, povidone, shellac, simethicone, silica, sodium alginate, sodium benzoate, sodium bicarbonate, stearic acid, sucrose, talc, titanium dioxide, triacetin, triethyl citrate, wax.
| BET-R-PREP BISACODYL
bisacodyl tablet |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Satius Pharmaceuticals, LLC (080518631) |
Revised: 10/2023
Document Id: 071dcae0-3041-2684-e063-6394a90a33b2
Set id: c222dc49-3081-4b4f-b1f3-8c60a86456dc
Version: 5
Effective Time: 20231007
Satius Pharmaceuticals, LLC