Label: MULTIMIN 90- zinc oxide, manganese carbonate, sodium selenite, copper carbonate injection, solution
- NDC Code(s): 49920-006-01, 49920-006-05
- Packager: Multimin North America, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
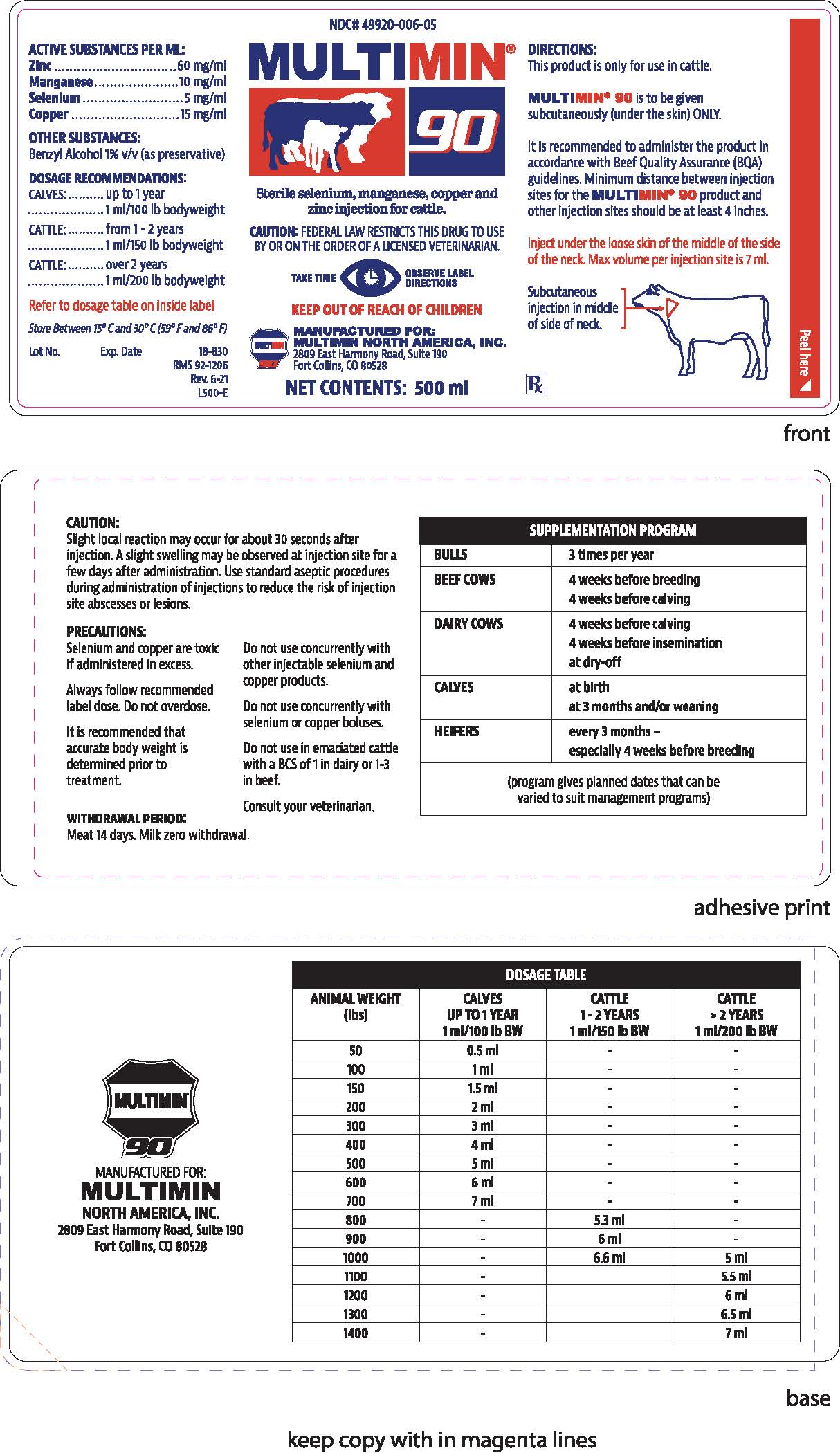
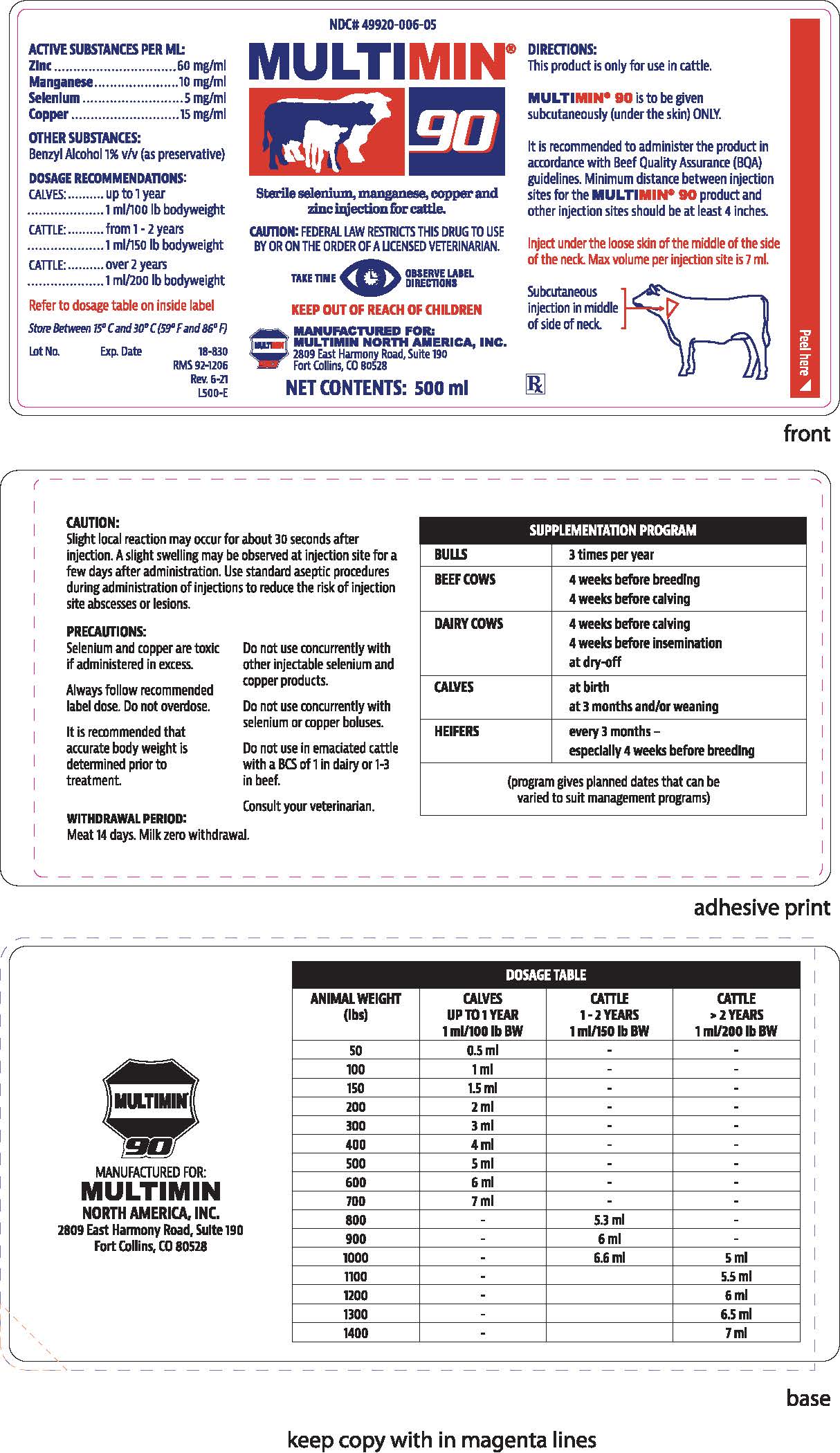
GUARANTEED ANALYSIS OF FEED
ACTIVE SUBSTANCES PER ML:
Zinc.................................................................................. 60 mg/ml
Manganese...................................................................... 10 mg/ml
Selenium............................................................................ 5 mg/ml
Copper............................................................................. 15 mg/mlOTHER SUBSTANCES:
Benzyl alcohol 1% v/v (as preservative) -
DOSAGE & ADMINISTRATION
DIRECTIONS:
This product is only for use in cattle.
MULTIMIN®90 is to be given
subcutaneously (under the skin) ONLY.It is recommended to administer the product in
accordance with Beef Quality Assurance (BQA)
guidelines. Minimum distance between injection
sites for the MULTIMIN®90 product and
other injection sites should be at least 4 inches.Inject under the loose skin of the middle of the side
of the neck. Max volume per injection site is 7ml.Subcutaneous injection in middle of side of neck.
RX
-
PRECAUTIONS
CAUTION:
Slight local reaction may occur for about 30 seconds after injection. A slight swelling may be observed at injection site for a few days after administration. Use standard aseptic procedures during administration of injections to reduce the risk of injection site abcesses or lesions.PRECAUTIONS:
Selenium and copper are toxic if administered in excess.
Always follow recommended label dose. Do not overdose.
It is recommended that accurate body weight is determined prior to treatment.
Do not use concurrently with other injectable selenium and copper products.
Do not use concurrently with selenium or copper boluses.
Do not use in emaciated cattle with a BCS of 1 in dairy or 1-3 in beef.
Consult your veterinarian.
WITHDRAWAL PERIOD:
Meat 14 days. Milk zero withdrawal.
- STORAGE AND HANDLING
-
DOSAGE & ADMINISTRATION
SUPPLEMENTATION PROGRAM BULLS 3 times per year BEEF COWS 4 weeks before breeding
4 weeks before calvingDAIRY COWS 4 weeks before calving
4 weeks before insemination
at dry-offCALVES at birth
at 3 months and/or weaningHEIFERS every 3 months -
especially 4 weeks before breeding
(program gives planned dates that can be varied to suit management programs) -
DOSAGE & ADMINISTRATION
MULTIMIN 90
MANUFACTURED FOR
MULTIMIN
NORTH AMERICA, INC.
2809 East Harmony Road, Suite 190
Fort Collins, CO 80528
DOSAGE TABLE ANIMAL WEIGHT
(lbs)
CALVES
UP TO 1 YEAR
1ml/100lb BW
CATTLE
1-2 YEARS
1ml/150lb BW
CATTLE
> 2 YEARS
1ml/200lb BW
50 0.5 ml - - 100 1 ml - - 150 1.5 ml - - 200 2 ml - - 300 3 ml - - 400 4 ml - - 500 5 ml - - 600 6 ml - - 700 7 ml - - 800 - 5.3 ml - 900 - 6 ml - 1000 - 6.6 ml 5 ml 1100 - 5.5 ml 1200 - 6 ml 1300 - 6.5 ml 1400 - 7 ml -
Package Label Principle Display Panel
NDC# 49920-006-01
MULTIMIN®90
Sterile selenium, manganese, copper and zinc injection for cattle
CAUTION: FEDERAL LAW RESTRICTS THIS DRUG TO USE BY OR ON THE ORDER OF A LICENSED VETERINARIAN.
TAKE TIME OBSERVE LABEL DIRECTIONS
KEEP OUT OF REACH OF CHILDREN
MULTIMIN NORTH AMERICA, INC.2809 East Harmony Road, Suite 190
Fort Collins, CO 80525
NET CONTENTS: 100 ml
DIRECTIONS:
This product is only for use in cattle.MULTIMIN®90 is to be given
subcutaneously (under the skin) ONLY.It is recommended to administer the product in
accordance with Beef Quality Assurance (BQA)
guidelines. Minimum distance between injection
sites for the MULTIMIN®90 product and
other injection sites should be at least 4 inches.Inject under the loose skin of the middle of the side
of the neck. Max volume per injection site is 7ml.Subcutaneous injection in middle of side of neck.
RX
ACTIVE SUBSTANCES PER ML:
Zinc.................................................................................. 60 mg/ml
Manganese...................................................................... 10 mg/ml
Selenium............................................................................ 5 mg/ml
Copper............................................................................. 15 mg/ml
OTHER SUBSTANCES:
Benzyl alcohol 1% v/v (as preservative)DOSAGE RECOMMENDATIONS:
CALVES: .........up to 1 year.........................1 ml/per 100 lb bodyweight
CATTLE: .........from 1-2 years......................1 ml/per 150 lb bodyweight
CATTLE: .........over 2 years.........................1 ml/per 200 lb bodyweightRefer to dosage table on inside label.
Store between 15°C and 30°C (59°F and 86°F)
Lot No. Exp. Date

-
Package Label Principle Display Panel
NDC# 49920-006-05
MULTIMIN®
90
Sterile selenium, manganese, copper and zinc injection for cattle
CAUTION: FEDERAL LAW RESTRICTS THIS DRUG TO USE BY OR ON THE ORDER OF A LICENSED VETERINARIAN.
TAKE TIME OBSERVE LABEL DIRECTIONS
KEEP OUT OF REACH OF CHILDREN
MULTIMIN NORTH AMERICA, INC.2809 East Harmony Road, Suite 190
Fort Collins, CO 80525
NET CONTENTS: 500 ml
DIRECTIONS:
This product is only for use in cattle.MULTIMIN®90 is to be given
subcutaneously (under the skin) ONLY.It is recommended to administer the product in
accordance with Beef Quality Assurance (BQA)
guidelines. Minimum distance between injection
sites for the MULTIMIN®90 product and
other injection sites should be at least 4 inches.Inject under the loose skin of the middle of the side
of the neck. Max volume per injection site is 7ml.Subcutaneous injection in middle of side of neck.
RX
ACTIVE SUBSTANCES PER ML:
Zinc.................................................................................. 60 mg/ml
Manganese...................................................................... 10 mg/ml
Selenium............................................................................ 5 mg/ml
Copper............................................................................. 15 mg/ml
OTHER SUBSTANCES:
Benzyl alcohol 1% v/v (as preservative)DOSAGE RECOMMENDATIONS:
CALVES: .........up to 1 year.........................1 ml/per 100 lb bodyweight
CATTLE: .........from 1-2 years......................1 ml/per 150 lb bodyweight
CATTLE: .........over 2 years.........................1 ml/per 200 lb bodyweightRefer to dosage table on inside label.
Store between 15°C and 30°C (59°F and 86°F)
Lot No. Exp. Date
-
INGREDIENTS AND APPEARANCE
MULTIMIN 90
zinc oxide, manganese carbonate, sodium selenite, copper carbonate injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:49920-006 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 60 mg in 1 mL MANGANESE CARBONATE (UNII: 9ZV57512ZM) (MANGANESE CATION (2+) - UNII:H6EP7W5457) MANGANESE CATION (2+) 10 mg in 1 mL SODIUM SELENITE (UNII: HIW548RQ3W) (SELENITE ION - UNII:KXO0259XJ1) SELENIUM 5 mg in 1 mL CUPRIC CARBONATE (UNII: 9AOA5F11GJ) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 15 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) EDETIC ACID (UNII: 9G34HU7RV0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49920-006-01 1 in 1 BOX 1 100 mL in 1 BOTTLE 2 NDC:49920-006-05 1 in 1 BOX 2 500 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/01/2009 Labeler - Multimin North America, Inc. (831737239) Registrant - Multimin North America, Inc. (831737239) Establishment Name Address ID/FEI Business Operations Nova-Tech 196078976 manufacture Establishment Name Address ID/FEI Business Operations Tairgi Tread-Lia Baile Na Sceilge Teoranta (DbA Ballinskelligs Veterinary Products [BVP] 986182178 manufacture, analysis Establishment Name Address ID/FEI Business Operations Quality Chemicals, S.L. 565078185 analysis Establishment Name Address ID/FEI Business Operations Purity Chemicals 465450491 api manufacture Establishment Name Address ID/FEI Business Operations Grillo Zincoxide GmBH 315419200 api manufacture