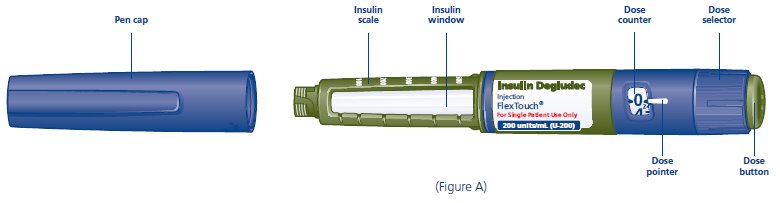
Label: INSULIN DEGLUDEC injection, solution
- NDC Code(s): 73070-400-11, 73070-403-15, 73070-503-15
- Packager: Novo Nordisk Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 1, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use INSULIN DEGLUDEC safely and effectively. See full prescribing information for INSULIN DEGLUDEC.
INSULIN DEGLUDEC injection, for subcutaneous use
Initial U.S. Approval: 2015
This product is Tresiba (insulin degludec).INDICATIONS AND USAGE
Insulin Degludec is a long-acting human insulin analog indicated to improve glycemic control in patients 1 year of age and older with diabetes mellitus (1).
Limitations of Use:
- •
- Not recommended for the treatment of diabetic ketoacidosis.
DOSAGE AND ADMINISTRATION
- •
- See Full Prescribing Information for important administration instructions (2.1).
- •
- Inject Insulin Degludec subcutaneously into the thigh, upper arm, or abdomen (2.1).
- •
- Rotate injection sites to reduce risk of lipodystrophy and localized cutaneous amyloidosis (2.1).
- •
- For pediatric patients requiring less than 5 units of Insulin Degludec each day, use an Insulin Degludec U-100 vial (2.1).
- •
- In adults, inject subcutaneously once daily at any time of day (2.2).
- •
- In pediatric patients inject subcutaneously once daily at the same time every day (2.2).
- •
- Individualize dose based on type of diabetes, metabolic needs, blood glucose monitoring results and glycemic control goal (2.2).
- •
- The recommended days between dose increases are 3 to 4 days (2.2).
- •
- See Full Prescribing Information for recommended starting dose in insulin naïve patients and patients already on insulin therapy (2.3, 2.4).
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- Never share an Insulin Degludec FlexTouch pen, insulin syringe, or needle between patients, even if the needle is changed (5.1).
- •
- Hyperglycemia or hypoglycemia with changes in insulin regimen: Make changes to a patient’s insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) under close medical supervision with increased frequency of blood glucose monitoring (5.2).
- •
- Hypoglycemia: May be life-threatening. Increase monitoring with changes to: insulin dosage, concomitant drugs, meal pattern, physical activity; and in patients with renal impairment or hepatic impairment or hypoglycemia unawareness (5.3, 5.4, 6.1).
- •
- Hypoglycemia due to medication errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection. DO NOT transfer Insulin Degludec from the Insulin Degludec pen into a syringe for administration as overdosage and severe hypoglycemia can result (5.4).
- •
- Hypersensitivity reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Insulin Degludec, monitor and treat if indicated (5.5).
- •
- Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk for hypokalemia and treat if indicated (5.6).
- •
- Fluid retention and heart failure with concomitant use of Thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs (5.7).
ADVERSE REACTIONS
Adverse reactions commonly associated with Insulin Degludec are:
- •
- hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema and weight gain (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Pharma, Inc. at 1-800-727-6500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 General Dosing Instructions
2.3 Starting Dose in Insulin Naïve Patients
2.4 Switching to Insulin Degludec from Other Insulin Therapies
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Never Share an Insulin Degludec FlexTouch Pen, Needle, or Insulin Syringe Between Patients
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
5.3 Hypoglycemia
5.4 Hypoglycemia Due to Medication Errors
5.5 Hypersensitivity Reactions
5.6 Hypokalemia
5.7 Fluid Retention and Congestive Heart Failure with Concomitant Use of a PPAR Gamma Agonist
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Type 1 Diabetes – Adult
14.2 Type 1 Diabetes – Pediatric Patients 1 Year of Age and Older
14.3 Type 2 Diabetes - Adult
14.4 Safety Outcomes Trial
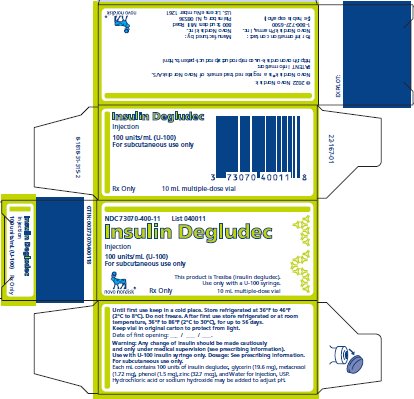
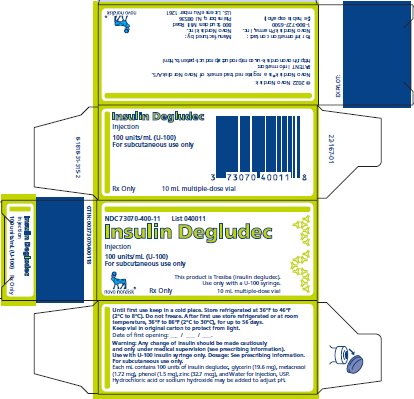
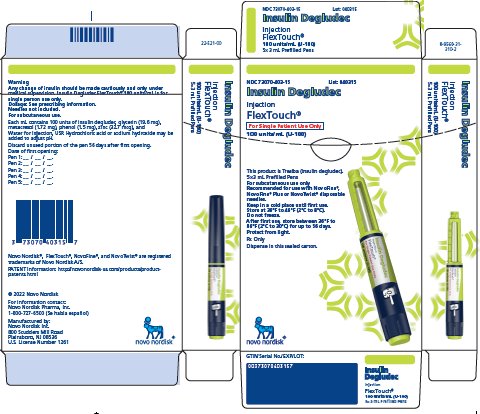
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Recommended Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
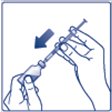
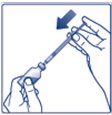
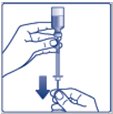
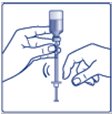
- •
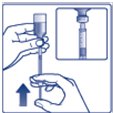
- Always check insulin labels before administration. This product is Tresiba (insulin degludec) [see Warnings and Precautions (5.4)].
- •
- Inspect visually for particulate matter and discoloration. Only use Insulin Degludec if the solution appears clear and colorless.
- •
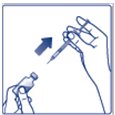
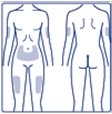
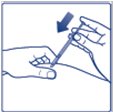
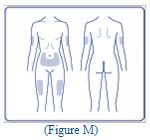
- Inject Insulin Degludec subcutaneously into the thigh, upper arm, or abdomen.
- •
- Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2), Adverse Reactions (6.1, 6.3)].
- •
- During changes to a patient’s insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
- •
- For pediatric patients requiring less than 5 units of Insulin Degludec each day, use the Insulin Degludec U-100 vial.
- •
- DO NOT administer Insulin Degludec intravenously or in an insulin infusion pump.
- •
- DO NOT dilute or mix Insulin Degludec with any other insulin or solution.
- •
- DO NOT transfer Insulin Degludec from the Insulin Degludec FlexTouch pen into a syringe for administration [see Warnings and Precautions (5.4)].
- •
- Use Insulin Degludec FlexTouch pens with caution in patients with visual impairment that may rely on audible clicks to dial their dose.
2.2 General Dosing Instructions
- •
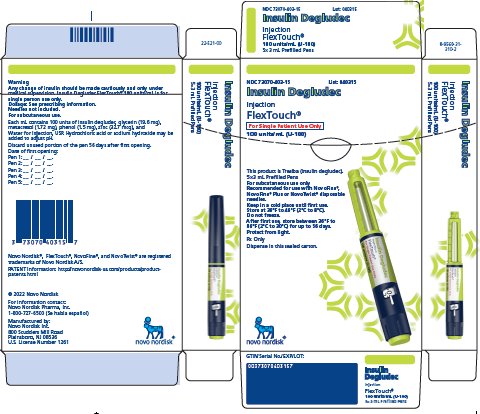
- Insulin Degludec is available in 2 concentrations (U-100 and U-200):
- o
- Insulin Degludec U-100 is available, as a single-patient use FlexTouch pen and multiple-dose vial.
- ▪
- The FlexTouch pen delivers doses in 1 unit increments and can deliver up to 80 units in a single injection.
- o
- Insulin Degludec U-200 is available as a single-patient-use FlexTouch pen.
- ▪
- The FlexTouch pen delivers doses in 2 unit increments and can deliver up to 160 units in a single injection.
- •
- DO NOT perform dose conversion when using the Insulin Degludec U-100 or U-200 FlexTouch pens. The dose window shows the number of insulin units to be delivered and no conversion is needed.
- •
- In adults, inject Insulin Degludec subcutaneously once-daily at any time of day.
- •
- In pediatric patients inject Insulin Degludec subcutaneously once-daily at the same time every day.
- •
- Individualize and titrate the dose of Insulin Degludec based on the patient’s metabolic needs, blood glucose monitoring results, and glycemic control goal.
- •
- The recommended days between dose increases are 3 to 4 days.
- •
- Dose adjustments may be needed with changes in physical activity, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness to minimize the risk of hypoglycemia or hyperglycemia [see Warnings and Precautions (5.3)].
- •
- For adult patients, instruct patients who miss a dose of Insulin Degludec to inject their daily dose during waking hours upon discovering the missed dose. Instruct patients to ensure that at least 8 hours have elapsed between consecutive Insulin Degludec injections.
- •
- For pediatric patients, instruct patients who miss a dose of Insulin Degludec to contact their healthcare provider for guidance and to monitor blood glucose levels more frequently until the next scheduled Insulin Degludec dose.
- •
- In patients with type 1 diabetes, Insulin Degludec must be used concomitantly with short-acting insulin.
2.3 Starting Dose in Insulin Naïve Patients
Recommended Starting Dosage in Patients with Type 1 Diabetes Mellitus:
The recommended starting dose of Insulin Degludec in insulin naïve patients with type 1 diabetes is approximately one-third to one-half of the total daily insulin dose. The remainder of the total daily insulin dose should be administered as a short-acting insulin and divided between each daily meal. As a general rule, 0.2 to 0.4 units of insulin per kilogram of body weight can be used to calculate the initial total daily insulin dose in insulin naïve patients with type 1 diabetes.
Recommended Starting Dosage in Patients with Type 2 Diabetes Mellitus:
The recommended starting dose of Insulin Degludec in insulin naïve patients with type 2 diabetes mellitus is 10 units once daily.
2.4 Switching to Insulin Degludec from Other Insulin Therapies
Dosage adjustments are recommended to lower the risk of hypoglycemia when switching patients to Insulin Degludec from another insulin therapy [see Warnings and Precautions (5.3)].
Adults withType 1 or Type 2 Diabetes Mellitus:
Start Insulin Degludec at the same unit dose as the total daily long or intermediate-acting insulin unit dose.
Pediatric Patients 1 Year of Age and Older with Type 1 or Type 2 Diabetes Mellitus:
Start Insulin Degludec at 80% of the total daily long or intermediate-acting insulin unit dose to minimize the risk of hypoglycemia [see Warnings and Precautions (5.2)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Never Share an Insulin Degludec FlexTouch Pen, Needle, or Insulin Syringe Between Patients
Insulin Degludec FlexTouch disposable prefilled pens should never be shared between patients, even if the needle is changed. Patients using Insulin Degludec vials should never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6.1, 6.3)].
Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes, adjustments in concomitant anti-diabetic treatment may be needed [see Dosage and Administration (2.4)].
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction of insulin, including Insulin Degludec [see Adverse Reactions (6.1)]. Severe hypoglycemia can cause seizures, may be life-threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place the patient and others at risk in situations where these abilities are important (e.g., driving or operating other machinery). Insulin Degludec, or any insulin, should not be used during episodes of hypoglycemia [see Contraindications (4)].
Hypoglycemia can happen suddenly and symptoms may differ in each patient and change over time in the same patient. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic neuropathy, using drugs that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or who experience recurrent hypoglycemia.
The long-acting effect of Insulin Degludec may delay recovery from hypoglycemia compared to shorter-acting insulins.
Risk Factors for Hypoglycemia
The risk of hypoglycemia generally increases with intensity of glycemic control. The risk of hypoglycemia after an injection is related to the duration of action of the insulin [see Clinical Pharmacology (12.2)] and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of Insulin Degludec may vary among different patients or at different times in the same patients and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature.
Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to concomitant drugs [see Drug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products, have been reported. To avoid medication errors between Insulin Degludec and other insulins, instruct patients to always check the insulin label before each injection.
To avoid dosing errors and potential overdose, never use a syringe to remove Insulin Degludec from the Insulin Degludec FlexTouch disposable insulin prefilled pen [see Dosage and Administration (2.1) and Warnings and Precautions (5.3)].
5.5 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including Insulin Degludec [see Adverse Reactions (6.1)]. If hypersensitivity reactions occur, discontinue Insulin Degludec; treat per standard of care and monitor until symptoms and signs resolve. Insulin Degludec is contraindicated in patients who have had hypersensitivity reactions to insulin degludec or any of the excipients.
5.6 Hypokalemia
All insulins, including Insulin Degludec, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
5.7 Fluid Retention and Congestive Heart Failure with Concomitant Use of a PPAR Gamma Agonist
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists can cause dose related fluid retention, when used in combination with insulin. Fluid retention may lead to or exacerbate congestive heart failure. Patients treated with insulin, including Insulin Degludec and a PPAR-gamma agonist should be observed for signs and symptoms of congestive heart failure. If congestive heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
-
6 ADVERSE REACTIONS
The following adverse reactions are also discussed elsewhere:
- •
- Hypoglycemia [see Warnings and Precautions (5.3)]
- •
- Hypoglycemia due to Medication errors [see Warnings and Precautions (5.4)]
- •
- Hypersensitivity reactions [see Warnings and Precautions (5.5)]
- •
- Hypokalemia [see Warnings and Precautions (5.6)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of Insulin Degludec in subjects with type 1 diabetes or type 2 diabetes was evaluated in nine trials of 6-12 month duration in adults and in one trial of 12-month duration in pediatric patients 1 year of age and older with type 1 diabetes. The cardiovascular safety of Insulin Degludec was evaluated in one double-blinded, event-driven trial of 2-year median duration in patients with type 2 diabetes at high risk of cardiovascular events [see Clinical Studies (14)].
The data in Table 1 reflect the exposure of 1102 adults with type 1 diabetes to Insulin Degludec with a mean exposure duration to Insulin Degludec of 34 weeks in three open-label trials; Study A, B and C [see Clinical Studies (14.1)]. The mean age was 43 years and 1% were older than 75 years. Fifty-seven percent were male, 81% were White, 2% were Black or African American and 4% were Hispanic. The mean body mass index (BMI) was 26 kg/m2. The mean duration of diabetes was 18 years and the mean HbA1c at baseline was 7.8%. A history of neuropathy, ophthalmopathy, nephropathy and cardiovascular disease at baseline was reported in 11%, 16%, 7% and 0.5% respectively. The mean eGFR at baseline was 87 mL/min/1.73 m2 and 7% of the patients had an eGFR less than 60 mL/min/1.73 m2.
The data in Table 2 reflect the exposure of 2713 adults with type 2 diabetes to Insulin Degludec with a mean exposure duration to Insulin Degludec of 36 weeks in six open-label trials; Study D, E, F, G, H and I [see Clinical Studies (14.3)]. The mean age was 58 years and 3% were older than 75 years. Fifty-eight percent were male, 71% were White, 7% were Black or African American and 13% were Hispanic. The mean BMI was 30 kg/m2. The mean duration of diabetes was 11 years and the mean HbA1c at baseline was 8.3%. A history of neuropathy, ophthalmopathy, nephropathy and cardiovascular disease at baseline was reported for 14%, 10%, 6% and 0.6% of participants respectively. At baseline, the mean eGFR was 83 mL/min/1.73 m2 and 9% had an eGFR less than 60 mL/min/1.73 m2.
Common adverse reactions (excluding hypoglycemia) occurring in Insulin Degludec treated subjects during clinical trials in adult patients with type 1 diabetes mellitus and adults with type 2 diabetes mellitus are listed in Table 1 and Table 2, respectively. Common adverse reactions were defined as reactions occurring in ≥5% of the population studied. Hypoglycemia is not shown in these tables but discussed in a dedicated subsection below.
174 pediatric patients 1 year of age and older with type 1 diabetes were exposed to Insulin Degludec with a mean exposure to Insulin Degludec of 48 weeks. The mean age was 10 years: 25% were ages 1-5 years, 40% were ages 6-11 years, and 35% were ages 12-17 years. 55% were male, 78% were White, 3% were Black or African American and 4% were Hispanic. The mean body mass index (BMI) was 18.7 kg/m2. The mean duration of diabetes was 3.9 years and the mean HbA1c at baseline was 8.2%. Common adverse reactions in Insulin Degludec treated pediatric patients with type 1 diabetes mellitus were similar to the adverse reactions listed in Table 1.
Table 1: Adverse Reactions Occurring in ≥5% of Insulin Degludec-Treated Adult Patients with Type 1 Diabetes Mellitus
Adverse Reaction
Insulin Degludec
(N=1,102)
Nasopharyngitis
23.9 %
Upper respiratory tract infection
11.9 %
Headache
11.8 %
Sinusitis
5.1 %
Gastroenteritis
5.1 %
Table 2: Adverse Reactions Occurring in ≥5% of Insulin Degludec-Treated Adult Patients with Type 2 Diabetes Mellitus
Adverse Reaction
Insulin Degludec
(N=2,713)
Nasopharyngitis
12.9 %
Headache
8.8 %
Upper respiratory tract infection
8.4 %
Diarrhea
6.3 %
Hypoglycemia
Hypoglycemia was the most commonly observed adverse reaction in patients treated with Insulin Degludec. The rates of reported hypoglycemia depended on the definition of hypoglycemia used, diabetes type, insulin dose, intensity of glucose control, background therapies, and other intrinsic and extrinsic patient factors. For these reasons, comparing rates of hypoglycemia in clinical trials for Insulin Degludec with the incidence of hypoglycemia for other products may be misleading and also, may not be representative of hypoglycemia rates that will occur in clinical practice.
In the open-label adult clinical trials of patients with type 1 and type 2 diabetes, and in the open-label pediatric clinical trial of patients with type 1 diabetes, percentages of adult and pediatric patients with type 1 diabetes randomized to Insulin Degludec who experienced at least one episode of hypoglycemia in clinical trials [see Clinical Studies (14)] and adults with type 2 diabetes are shown in Tables 3 and 4, respectively.
Severe hypoglycemia in the open-label trials with adult patients was defined as an episode requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. Severe hypoglycemia in the pediatric trial was defined as an altered mental status where the child could not assist in his own care, was semiconscious or unconscious, or in a coma ± convulsions and may require parenteral therapy (glucagon or intravenous glucose). A hypoglycemia episode was defined as a severe hypoglycemia episode or an episode where a laboratory or a self-measured glucose calibrated to plasma was less than 56 mg/dL or where a whole blood glucose was less than 50 mg/dL (i.e., with or without the presence of hypoglycemic symptoms).
Table 3: Percent (%) of Type 1 Diabetes Patients Experiencing at Least One Episode of Severe Hypoglycemia or Hypoglycemia§ on Insulin Degludec in Open-Label Adult and Pediatric Clinical Trials
Study A
Adults
+ insulin aspart
52 weeksStudy B
Adults
+ insulin aspart
26 weeksStudy C
Adults
+ insulin aspart
26 weeksStudy J
Pediatrics
+ insulin aspart
52 weeksInsulin Degludec
(N=472)Insulin Degludec
(N=301)Insulin Degludec at the same time each day
(N=165)Insulin Degludec at alternating times
(N=164)Insulin Degludec
(N=174)Severe hypoglycemia*
Percent of patients
12.3%
10.6%
12.7%
10.4%
17.8%
Hypoglycemia§
Percent of patients
95.6%
93.0%
99.4%
93.9%
98.3%
*Severe hypoglycemia in pediatric patients: an episode with altered mental status, where the child could not assist in his own care, was semiconscious or unconscious, or in a coma ± convulsions and may require parenteral therapy (glucagon or intravenous glucose).
§Hypoglycemia: a severe hypoglycemia episode or an episode where a laboratory or a self-measured glucose calibrated to plasma was less than 56 mg/dL or where a whole blood glucose was less than 50 mg/dL (i.e., with or without the presence of hypoglycemic symptoms).
Table 4: Percent (%) of Patients with Type 2 Diabetes Experiencing at Least One Episode of Severe Hypoglycemia or Hypoglycemia§ on Insulin Degludec in Open-Label Adult Clinical Trials
Study D
+ 1-2 OADs*
insulin naïve
52 weeksStudy E
+ 1-2 OADs*
insulin naïve
26 weeksStudy F
± 1-3 OADs*
insulin naïve
26 weeksStudy G
T2DM ± 0-3 OADs*
26 weeksStudy H
T2DM ± 0-2
OADs* +
insulin aspart
52 weeksStudy I
T2DM ± 1-2
OADs*
insulin naïve
26 weeksInsulin Degludec
(N=766)Insulin Degludec
(N=228)Insulin Degludec
(N=284)Insulin Degludec
(N=226)Insulin Degludec (alternating
time)
(N=230)Insulin Degludec
(N=753)Insulin Degludec
(N=226)Severe Hypoglycemia
Percent of patients
0.3%
0
0
0.9%
0.4%
4.5%
0.4%
Hypoglycemia§
Percent of patients
46.5%
28.5%
50%
43.8%
50.9%
80.9%
42.5%
*OAD: oral antidiabetic agent, §Hypoglycemia: a severe hypoglycemia episode or an episode where a laboratory or a self-measured glucose calibrated to plasma was less than 56 mg/dL or where a whole blood glucose was less than 50 mg/dL (i.e., with or without the presence of hypoglycemic symptoms).
Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, generalized skin reactions, angioedema, bronchospasm, hypotension, and shock have occurred with insulin, including Insulin Degludec and may be life threatening. Hypersensitivity (manifested with swelling of tongue and lips, diarrhea, nausea, tiredness, and itching) and urticaria were reported in 0.9% of patients treated with Insulin Degludec.
Lipodystrophy
Long-term use of insulin, including Insulin Degludec, can cause lipodystrophy at the site of repeated insulin injections. Lipodystrophy includes lipohypertrophy (thickening of adipose tissue) and lipoatrophy (thinning of adipose tissue) and may affect insulin absorption [see Dosage and Administration (2.1)]. In the clinical program, lipodystrophy, lipohypertrophy, or lipoatrophy was reported in 0.3% of patients treated with Insulin Degludec.
Injection Site Reactions
Patients taking Insulin Degludec may experience injection site reactions, including injection site hematoma, pain, hemorrhage, erythema, nodules, swelling, discoloration, pruritus, warmth, and injection site mass. In the clinical program, injection site reactions occurred in 3.8% of patients treated with Insulin Degludec.
Weight Gain
Weight gain can occur with insulin therapy, including Insulin Degludec, and has been attributed to the anabolic effects of insulin. In the clinical program after 52 weeks of treatment, patients with type 1 diabetes treated with Insulin Degludec gained an average of 1.8 kg and patients with type 2 diabetes treated with Insulin Degludec gained an average of 3.0 kg.
Peripheral Edema
Insulin Degludec may cause sodium retention and edema. In the clinical program, peripheral edema occurred in 0.9% of patients with type 1 diabetes mellitus and 3.0% of patients with type 2 diabetes mellitus treated with Insulin Degludec.
6.2 Immunogenicity
As with all therapeutic proteins, insulin administration may cause anti-insulin antibodies to form. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay and may be influenced by several factors such as: assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to Insulin Degludec with the incidence of antibodies in other studies or to other products may be misleading.
In a 52-week trial of adult insulin-experienced type 1 diabetes patients, 68.9% of patients who received Insulin Degludec were positive at baseline for anti-insulin degludec antibodies and 12.3% of the patients developed anti-insulin degludec antibodies at least once during the trial. In a 52-week trial of pediatric insulin-experienced type 1 diabetes patients, 84.1% of patients who received Insulin Degludec were positive at baseline for anti-insulin degludec antibodies and 5.8% of patients developed anti-insulin degludec antibodies at least once during the trial. In a 52-week trial of adult insulin-naïve type 2 diabetes patients, 1.7% of patients who received Insulin Degludec were positive at baseline for anti-insulin degludec antibodies and 6.2% of patients developed anti-insulin degludec antibodies at least once during the trial. In these trials, between 96.7% and 99.7% of patients who were positive for anti-insulin degludec antibodies were also positive for anti-human insulin antibodies.
6.3 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of Insulin Degludec. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
-
7 DRUG INTERACTIONS
Table 5 includes clinically significant drug interactions with Insulin Degludec.
Table 5: Clinically Significant Drug Interactions with Insulin Degludec
- Drugs That May Increase the Risk of Hypoglycemia
- Drugs:
- Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analogs (e.g., octreotide), and sulfonamide antibiotics, GLP-1 receptor agonists, DPP-4 inhibitors, SGLT-2 inhibitors.
- Intervention:
- Dosage reductions and increased frequency of glucose monitoring may be required when Insulin Degludec is co-administered with these drugs.
- Drugs That May Decrease the Blood Glucose Lowering Effect of Insulin Degludec
- Drugs:
- Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones.
- Intervention:
- Dosage increases and increased frequency of glucose monitoring may be required when Insulin Degludec is co-administered with these drugs.
- Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of Insulin Degludec
- Drugs:
- Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia.
- Intervention:
- Dosage adjustment and increased frequency of glucose monitoring may be required when Insulin Degludec is co-administered with these drugs.
- Drugs That May Blunt Signs and Symptoms of Hypoglycemia
- Drugs:
- Beta-blockers, clonidine, guanethidine, and reserpine
- Intervention:
- Increased frequency of glucose monitoring may be required when Insulin Degludec is co-administered with these drugs.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from one unpublished trial and the published literature with Insulin Degludec use during pregnancy have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In a randomized, parallel-group, open-label actively controlled clinical trial that included 91 pregnant women with type 1 diabetes who were administered Insulin Degludec once daily and insulin aspart, beginning in gestational weeks 8 to 13 or prior to conception, no clear evidence of maternal or fetal risk associated with Insulin Degludec use was observed (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations).
Rats and rabbits were exposed to insulin degludec in animal reproduction studies during organogenesis. Pre-and post-implantation losses and visceral/skeletal abnormalities were observed in rats at doses 5 times (rat) and at 10 times (rabbit) the human exposure at a dose of 0.75 U/kg/day. These effects were similar to those observed in rats administered human insulin (NPH) (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively. The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational diabetes with a peri-conceptional HbA1c >7 and has been reported to be as high as 20 to 25% in women with a peri-conceptional HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/fetal Risk
Hypoglycemia and hyperglycemia occur more frequently during pregnancy in patients with pre-gestational diabetes. Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, still birth, and macrosomia related morbidity.
Data
Human Data
In an open-label clinical trial, 185 pregnant females with type 1 diabetes were treated with either Insulin Degludec (once daily) or insulin detemir (once or twice daily); both groups received insulin aspart 2 to 4 times daily with meals. There were no significant drug-associated differences in pregnancy outcomes or the health of the fetus and newborn between the two groups. In this study, the proportion of subjects with severe hypoglycemia and hypoglycemia was similar between the two treatment arms; for the definitions of severe hypoglycemia and hypoglycemia [see Adverse Reactions (6.1)]. Poor glucose control during pregnancy in both groups and small sample size were limitations of the study.
In about two thirds of infants, insulin degludec was detected in the infant cord blood at levels above the lower level of quantification of the assay.
Animal Data
Insulin degludec was investigated in studies covering fertility, embryo-fetal development and pre- and post-natal development in rats and during the period of embryo-fetal development in rabbits. Human insulin (NPH insulin) was included as comparator. In these studies, insulin degludec caused pre- and post-implantation losses and visceral/skeletal abnormalities when given subcutaneously at up to 21 U/kg/day in rats and 3.3 U/kg/day in rabbits, resulting in 5 times (rat) and 10 times (rabbit) the human exposure (AUC) at a human subcutaneous dose of 0.75 U/kg/day. Overall, the effects of insulin degludec were similar to those observed with human insulin, which were probably secondary to maternal hypoglycemia.
8.2 Lactation
Risk Summary
There are no data on the presence of insulin degludec in human milk, the effects on the breastfed infant, or the effects on milk production. Insulin degludec is present in rat milk [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Insulin Degludec and any potential adverse effects on the breastfed infant from Insulin Degludec or from the underlying maternal condition.
Data
In lactating rats, insulin degludec was present in milk at a concentration lower than that in plasma.
8.4 Pediatric Use
The safety and effectiveness of Insulin Degludec to improve glycemic control in pediatric patients 1 year of age or older with diabetes mellitus have been established. The use of Insulin Degludec for this indication is supported by evidence from an adequate and well-controlled trial and a pharmacokinetic study (trials included pediatric patients 1 year of age and older with type 1 diabetes mellitus) [see Clinical Pharmacology (12.3) and Clinical Studies (14.2)]. The use of Insulin Degludec in pediatric patients 1 year of age and older with type 2 diabetes mellitus is also supported by evidence from adequate and well-controlled trials in adults with type 2 diabetes mellitus [see Clinical Studies (14.3)].
In pediatric patients 1 year of age and older already on insulin therapy, start Insulin Degludec at a reduced dose to minimize the risk of hypoglycemia [see Dosage and Administration (2.4)].
The safety and effectiveness of Insulin Degludec have not been established in pediatric patients less than 1-year-old.
8.5 Geriatric Use
In controlled clinical trials [see Clinical Studies (14)] a total of 77 (7%) of the 1102 Insulin Degludec-treated patients with type 1 diabetes were 65 years or older and 9 (1%) were 75 years or older. A total of 670 (25%) of the 2713 Insulin Degludec-treated patients with type 2 diabetes were 65 years or older and 80 (3%) were 75 years or older. Differences in safety or effectiveness were not suggested in subgroup analyses comparing subjects older than 65 years to younger subjects.
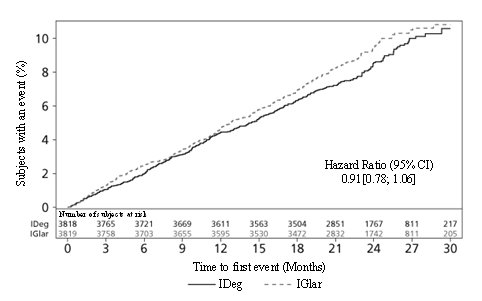
In the safety outcomes trial (DEVOTE), a total of 1983 (52%) of the 3818 Insulin Degludec-treated patients with type 2 diabetes were 65 years or older and 381 (10%) were 75 years or older. Differences in safety or effectiveness were not observed in these subgroup analyses.
Nevertheless, greater caution should be exercised when Insulin Degludec is administered to geriatric patients since greater sensitivity of some older individuals to the effects of Insulin Degludec cannot be ruled out. The initial dosing, dose increments, and maintenance dosage should be conservative to avoid hypoglycemia. Hypoglycemia may be more difficult to recognize in the geriatric patients.
8.6 Renal Impairment
In clinical trials [see Clinical Studies (14)] a total of 75 (7%) of the 1102 Insulin Degludec-treated patients with type 1 diabetes had an eGFR less than 60 mL/min/1.73 m2 and 1 (0.1%) had an eGFR less than 30 mL/min/1.73 m2. A total of 250 (9%) of the 2713 Insulin Degludec-treated patients with type 2 diabetes had an eGFR less than 60 mL/min/1.73 m2 and no subjects had an eGFR less than 30 mL/min/1.73 m2.
In the safety outcomes trial (DEVOTE), a total of 1429 (37.4%) of the 3818 Insulin Degludec-treated patients with type 2 diabetes had an eGFR less than 60 mL/min/1.73 m2, and 108 (2.8%) subjects had an eGFR less than 30 mL/min/1.73 m2. Differences in safety or effectiveness were not observed in the subgroup analyses.
No clinically relevant difference in the pharmacokinetics of Insulin Degludec was identified in a study comparing healthy subjects and subjects with kidney impairment including subjects with end stage kidney disease [see Clinical Pharmacology (12.3)]. However, as with all insulin products, glucose monitoring should be intensified and the Insulin Degludec dosage adjusted on an individual basis in patients with kidney impairment.
8.7 Hepatic Impairment
No difference in the pharmacokinetics of Insulin Degludec was identified in a study comparing healthy subjects and subjects with hepatic impairment (mild, moderate, and severe hepatic impairment) [see Clinical Pharmacology (12.3)]. However, as with all insulin products, glucose monitoring should be intensified and the Insulin Degludec dosage adjusted on an individual basis in patients with hepatic impairment.
-
10 OVERDOSAGE
An excess of insulin relative to food intake, energy expenditure, or both may lead to severe and sometimes prolonged and life-threatening hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.6)]. Mild episodes of hypoglycemia usually can be treated with oral glucose. Lowering the insulin dosage, and adjustments in meal patterns or exercise may be needed. More severe episodes of hypoglycemia with coma, seizure, or neurologic impairment may be treated with a glucagon for emergency use or concentrated intravenous glucose. After apparent clinical recovery from hypoglycemia, continued observation and additional carbohydrate intake may be necessary to avoid reoccurrence of hypoglycemia. Hypokalemia must be corrected appropriately.
-
11 DESCRIPTION
Insulin degludec is a long-acting basal human insulin analog for subcutaneous injection produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae followed by chemical modification.
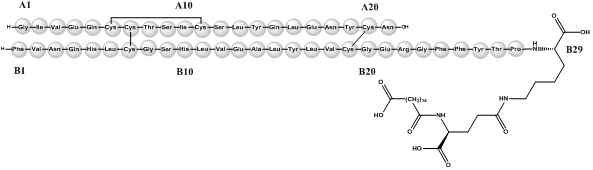
Insulin degludec differs from human insulin in that the amino acid threonine in position B30 has been omitted and a side-chain consisting of glutamic acid and a C16 fatty acid has been attached (chemical name: LysB29(Nε-hexadecandioyl-γ-Glu) des(B30) human insulin). Insulin degludec has a molecular formula of C274H411N65O81S6 and a molecular weight of 6.104 kDa. It has the following structure:
Figure 1: Structural Formula of Insulin Degludec
Insulin Degludec injection is a sterile, aqueous, clear, and colorless solution available as 100 units/mL (U-100) or 200 units/mL (U-200) for subcutaneous use.
For the 100 units/mL solution, each mL contains 100 units of insulin degludec and glycerin (19.6 mg), metacresol (1.72 mg), phenol (1.5 mg), zinc (32.7 mcg), and Water for Injection, USP.
For the 200 units/mL solution, each mL contains 200 units of insulin degludec and glycerin (19.6 mg), metacresol (1.72 mg), phenol (1.5 mg), zinc (71.9 mcg), and Water for Injection, USP.
Insulin Degludec has a pH of approximately 7.6. Hydrochloric acid or sodium hydroxide may be added to adjust pH.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary activity of insulin, including Insulin Degludec, is regulation of glucose metabolism. Insulin and its analogs lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin also inhibits lipolysis and proteolysis, and enhances protein synthesis. Insulin Degludec forms multi-hexamers when injected into the subcutaneous tissue resulting in a subcutaneous insulin degludec depot. The protracted time action profile of Insulin Degludec is predominantly due to delayed absorption of insulin degludec from the subcutaneous tissue to the systemic circulation and to a lesser extent due to binding of insulin-degludec to circulating albumin.
12.2 Pharmacodynamics
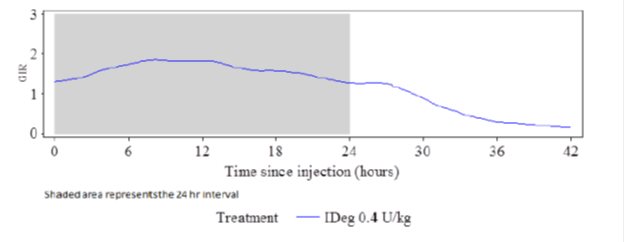
The glucose-lowering effect of Insulin Degludec after 8 days of once-daily dosing was measured in a euglycemic glucose clamp study enrolling 21 patients with type 1 diabetes. Figure 2 shows the pharmacodynamic effect of Insulin Degludec over time following 8 once-daily subcutaneous injections of 0.4 U/kg of Insulin Degludec in patients with type 1 diabetes.
Figure 2: Mean GIR Profile for 0.4 units/kg Dose of Insulin Degludec (Steady State) in Patients with Type 1 Diabetes Mellitus
The mean maximum glucose lowering effect (GIRmax) of a 0.4 units/kg dose of Insulin Degludec was 2.0 mg/kg/min, which was observed at a median of 12 hours post-dose. The glucose lowering effect of Insulin Degludec lasted at least 42 hours after the last of 8 once-daily injections.
In patients with type 1 diabetes mellitus, the steady-state, within subjects, day-to-day variability in total glucose lowering effect was 20% with Insulin Degludec (within-subject coefficient of variation for AUCGIR,τ,SS).
The total glucose-lowering effect of Insulin Degludec over 24 hours measured in a euglycemic clamp study after 8 days of once-daily administration in patients with type 1 diabetes increases approximately in proportion to the dose for doses between 0.4 units/kg to 0.8 units/kg.
The total glucose-lowering effect of 0.4 units/kg of Insulin Degludec U-100 and 0.4 units/kg of Insulin Degludec U-200, administered at the same dose, and assessed over 24 hours in a euglycemic clamp study after 8 days of once-daily injection was comparable.
12.3 Pharmacokinetics
Absorption
In patients with type 1 diabetes, after 8 days of once daily subcutaneous dosing with 0.4 units/kg of Insulin Degludec, maximum degludec concentrations of 4472 pmol/L were attained at a median of 9 hours (tmax). After the first dose of Insulin Degludec, median onset of appearance was around one hour.
Total insulin degludec concentration (i.e., exposure) increased in a dose proportional manner after subcutaneous administration of 0.4 units/kg to 0.8 units/kg Insulin Degludec. Total and maximum insulin degludec exposure at steady state are comparable between Insulin Degludec U-100 and Insulin Degludec U-200 when each is administered at the same units/kg dose.
Insulin degludec concentration reached steady state levels after 3-4 days of Insulin Degludec administration [see Dosage and Administration (2.2)].
Distribution
The affinity of insulin degludec to serum albumin corresponds to a plasma protein binding of >99% in human plasma. The results of the in vitro protein binding studies demonstrate that there is no clinically relevant interaction between insulin degludec and other protein bound drugs.
Elimination
The half-life after subcutaneous administration is determined primarily by the rate of absorption from the subcutaneous tissue. On average, the half-life at steady state is approximately 25 hours independent of dose. Degradation of Insulin Degludec is similar to that of insulin human; all metabolites formed are inactive. The mean apparent clearance of insulin degludec is 0.03 L/kg (2.1 L/h in 70 kg patient) after single subcutaneous dose of 0.4 units/kg.
Specific Populations
Pediatrics-
Population pharmacokinetic analysis was conducted for Insulin Degludec using data from 199 pediatric subjects (1 to <18 years of age) with type 1 diabetes. Body weight was a significant covariate affecting the clearance of Insulin Degludec. After adjusting for body weight, the total exposure of Insulin Degludec at steady state was independent of age.
Geriatrics-
Pharmacokinetic and pharmacodynamic response of Insulin Degludec was compared in 13 younger adult (18−35 years) and 14 geriatric (≥65 years) subjects with type 1 diabetes following two 6-day periods of once-daily subcutaneous dosing with 0.4 units/kg dose of Insulin Degludec or insulin glargine. On average, the pharmacokinetic and pharmacodynamic properties of Insulin Degludec at steady-state were similar in younger adult and geriatric subjects, albeit with greater between subject variability among the geriatric subjects.
Gender-
The effect of gender on the pharmacokinetics of Insulin Degludec was examined in an across-trial analysis of the pharmacokinetic and pharmacodynamic studies conducted using unit/kg doses of Insulin Degludec. Overall, there were no clinically relevant differences in the pharmacokinetic properties of insulin degludec between female and male subjects.
Obesity-
The effect of BMI on the pharmacokinetics of Insulin Degludec was explored in a cross-trial analysis of pharmacokinetic and pharmacodynamic studies conducted using unit/kg doses of Insulin Degludec. For subjects with type 1 diabetes, no relationship between exposure of Insulin Degludec and BMI was observed. For subjects with type 1 and type 2 diabetes a trend for decrease in glucose-lowering effect of Insulin Degludec with increasing BMI was observed.
Race and Ethnicity-
Insulin Degludec has been studied in a pharmacokinetic and pharmacodynamic study in Black or African American subjects not of Hispanic or Latino origin (N=18), White subjects of Hispanic or Latino origin (N=22) and White subjects not of Hispanic or Latino origin (N=23) with type 2 diabetes mellitus conducted using unit/kg doses of Insulin Degludec. There were no statistically significant differences in the pharmacokinetic and pharmacodynamic properties of Insulin Degludec between the racial and ethnic groups investigated.
Pregnancy-
The effect of pregnancy on the pharmacokinetics and pharmacodynamics of Insulin Degludec has not been studied [see Use in Specific Populations (8.1)].
Renal Impairment-
Insulin Degludec pharmacokinetics was studied in 32 subjects (N=4-8/group) with normal or impaired renal function/end-stage renal disease following administration of a single subcutaneous dose (0.4 units/kg) of Insulin Degludec. Renal function was defined using creatinine clearance (Clcr) as follows: ≥90 mL/min (normal), 60-89 mL/min (mild), 30-59 mL/min (moderate) and <30 mL/min (severe). Subjects requiring dialysis were classified as having end-stage renal disease (ESRD). Total (AUCIDeg,0-120h,SD) and peak exposure of Insulin Degludec were on average about 10-25% and 13-27% higher, respectively in subjects with mild to severe renal impairment except subjects with ESRD who showed similar exposure as compared to subjects with normal renal function. No systematic trend was noted for this increase in exposure across different renal impairment subgroups. Hemodialysis did not affect clearance of Insulin Degludec (CL/FIDeg,SD) in subjects with ESRD [see Use in Specific Populations (8.6)].
Hepatic Impairment-
Insulin Degludec has been studied in a pharmacokinetic study in 24 subjects (N=6/group) with normal or impaired hepatic function (mild, moderate, and severe hepatic impairment) following administration of a single subcutaneous dose (0.4 units/kg) of Insulin Degludec. Hepatic function was defined using Child-Pugh Scores ranging from 5 (mild hepatic impairment) to 15 (severe hepatic impairment). No differences in the pharmacokinetics of Insulin Degludec were identified between healthy subjects and subjects with hepatic impairment [see Use in Specific Populations (8.7)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed to evaluate the carcinogenic potential of insulin degludec. In a 52-week study including human insulin (NPH insulin) as comparator (6.7 units/kg/day), Sprague-Dawley rats were dosed subcutaneously with insulin degludec at 3.3, 6.7, and 10 units/kg/day, resulting in 5 times the human exposure (AUC) when compared to a human subcutaneous dosage of 0.75 units/kg/day. Human insulin was dosed at 6.7 units/kg/day. No treatment-related increases in incidences of hyperplasia, benign or malignant tumors were recorded in female mammary glands from rats dosed with insulin degludec and no treatment related changes in the female mammary gland cell proliferation were found using BrdU incorporation. Further, no treatment related changes in the occurrence of hyperplastic or neoplastic lesions were seen in other tissues in animals dosed with insulin degludec when compared to vehicle or human insulin.
Genotoxicity testing of insulin degludec was not performed.
In a combined fertility and embryo-fetal study in male and female rats, treatment with insulin degludec up to 21 units/kg/day (approximately 5 times the human subcutaneous dose of 0.75 units/kg/day, based on units/body surface area) prior to mating and in female rats during gestation had no effect on mating performance and fertility.
-
14 CLINICAL STUDIES
The efficacy of Insulin Degludec administered once-daily either at the same time each day or at any time each day in patients with type 1 diabetes and used in combination with a mealtime insulin was evaluated in three randomized, open-label, treat-to-target, active-controlled trials in adults and one randomized, open-label, treat-to-target, active-controlled trial in pediatric patients 1 year of age and older. The efficacy of Insulin Degludec administered once-daily either at the same time each day or at any time each day in adult patients with type 2 diabetes and used in combination with a mealtime insulin or in combination with common oral anti-diabetic agents was evaluated in six randomized, open-label, treat-to-target active-controlled trials.
Adult patients treated with Insulin Degludec achieved levels of glycemic control similar to those achieved with LANTUS (insulin glargine 100 units/mL) and LEVEMIR (insulin detemir) and achieved statistically significant improvements compared to sitagliptin.
14.1 Type 1 Diabetes – Adult
Insulin Degludec Administered at the Same Time Each Day in Combination with a Rapid-Acting Insulin Analog at Mealtimes in Adult Patients
Study A
The efficacy of Insulin Degludec was evaluated in a 52-week randomized, open-label, multicenter trial in 629 patients with type 1 diabetes mellitus (Study A). Patients were randomized to Insulin Degludec once-daily with the evening meal or insulin glargine U-100 once-daily according to the approved labeling. Insulin aspart was administered before each meal in both treatment arms.
The mean age of the trial population was 43 years and mean duration of diabetes was 19 years. 59% were male. 93% were White, 2% Black or African American. 5% were Hispanic. 9% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 26.3 kg/m2.
At week 52, the difference in HbA1c reduction from baseline between Insulin Degludec and insulin glargine U-100 was -0.01% with a 95% confidence interval of [-0.14%; 0.11%] and met the pre-specified non-inferiority margin (0.4%). See Table 6, Study A.
Study B
The efficacy of Insulin Degludec was evaluated in a 26-week randomized, open-label, multicenter trial in 455 patients with type 1 diabetes mellitus (Study B). Patients were randomized to Insulin Degludec or insulin detemir once-daily in the evening. After 8 weeks, insulin detemir could be dosed twice-daily. 67% used insulin detemir once daily at end of trial. 33% used insulin detemir twice daily at end of trial. Insulin aspart was administered before each meal in both treatment arms.
The mean age of the trial population was 41 years and mean duration of diabetes was 14 years. 52% were male. 45% were White, 0.4% Black or African American. 4% were Hispanic. 4% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 23.9 kg/m2.
At week 26, the difference in HbA1c reduction from baseline between Insulin Degludec and insulin detemir was -0.09% with a 95% confidence interval of [-0.23%; 0.05%] and met the pre-specified non-inferiority margin (0.4%). See Table 6, Study B.
Table 6: Results at Week 52 in a Trial Comparing Insulin Degludec to Insulin Glargine U-100 (Study A) and Week 26 in a Trial Comparing Insulin Degludec to Insulin Detemir (Study B) in Adult Patients with Type 1 Diabetes Mellitus Receiving Insulin Aspart at Mealtimes
Study A
Study B
Insulin Degludec + Insulin aspart
Insulin glargine U-100 + Insulin aspart
Insulin Degludec + Insulin aspart
Insulin detemir + Insulin aspart
N
472
157
302
153
HbA1c (%)
Baseline
7.7
7.7
8.0
8.0
End of trial
7.3
7.3
7.3
7.3
Adjusted mean change from baseline*
-0.36
-0.34
-0.71
-0.61
Estimated treatment difference [95%CI]
Insulin Degludec - basal insulin U-100
-0.01 [-0.14;0.11]
-0.09 [-0.23;0.05]
Proportion Achieving HbA1c < 7% at Trial End
39.8%
42.7%
41.1%
37.3%
FPG (mg/dL)
Baseline
165
174
178
171
End of trial
141
149
131
161
Adjusted mean change from baseline
-27.6
-21.6
-43.3
-13.5
Daily basal insulin dose
Baseline mean
28 U
26 U
22 U
22 U
Mean dose at end of study
29 U1
31 U1
25 U2
29 U2
Daily bolus insulin dose
Baseline mean
29 U
29 U
28 U
31 U
Mean dose at end of study
32 U1
35 U1
36 U2
41 U2
- 1At Week 52
- 2At Week 26
- *The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study A, there were 14.8% of subjects in the Insulin Degludec and 11.5% Insulin glargine arms for whom data was missing at the time of the HbA1c measurement.
- In Study B, there were 6.3% of subjects in the Insulin Degludec and 9.8% Insulin detemir arms for whom data was missing at the time of the HbA1c measurement.
Study C: Insulin Degludec Administered at the Same Time Each Day or at Any Time Each Day in Combination with a Rapid-Acting Insulin Analog at Mealtimes in Adult Patients
The efficacy of Insulin Degludec was evaluated in a 26-week randomized, open-label, multicenter trial in 493 patients with type 1 diabetes mellitus. Patients were randomized to Insulin Degludec injected once-daily at the same time each day (with the main evening meal), to Insulin Degludec injected once daily at any time each day or to insulin glargine U-100 injected once-daily according to the approved labeling. The any time each day Insulin Degludec arm was designed to simulate a worst-case scenario injection schedule of alternating short and long, once daily, dosing intervals (i.e., alternating intervals of 8 to 40 hours between doses). Insulin Degludec in this arm was dosed in the morning on Monday, Wednesday, and Friday and in the evening on Tuesday, Thursday, Saturday, and Sunday. Insulin aspart was administered before each meal in all treatment arms.
The mean age of the trial population was 44 years and mean duration of diabetes was 19 years. 58% were male. 98% were White, 2% Black or African American. 3% were Hispanic. 7% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 26.7 kg/m2.
At week 26, the difference in HbA1c reduction from baseline between Insulin Degludec administered at alternating times and insulin glargine U-100 was 0.17% with a 95% confidence interval of [0.04%; 0.30%] and met the pre-specified non-inferiority margin (0.4%). See Table 7.
Table 7: Results at Week 26 in a Trial Comparing Insulin Degludec Dosed Once Daily at the Same and at Alternating Times Each Day to Insulin Glargine U-100 in Adult Patients with Type 1 Diabetes Mellitus Receiving Insulin Aspart at Mealtimes
Insulin Degludec at the same time each day + Insulin aspart
Insulin Degludec at alternating times + Insulin aspart
Insulin glargine U-100 + Insulin aspart
N
165
164
164
HbA1c (%)
Baseline
7.7
7.7
7.7
End of trial
7.3
7.3
7.1
Adjusted mean change from baseline*
-0.41
-0.40
-0.57
Estimated treatment difference [95%CI]
Insulin Degludec alternating - Insulin glargine U-100
0.17 [0.04;0.30]
Proportion Achieving HbA1c < 7% at Trial End
37.0%
37.2%
40.9%
FPG (mg/dL)
Baseline
179
173
175
End of trial
133
149
151
Adjusted mean change from baseline
-41.8
-24.7
-23.9
Daily basal insulin dose
Baseline mean
28 U
29 U
29 U
Mean dose at end of study
32 U
36 U
35 U
Daily bolus insulin dose
Baseline mean
29 U
33 U
32 U
Mean dose at end of study
27 U
30 U
35 U
*The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
In Study C, there were 15.8% and 15.9% of subjects in the Insulin Degludec (same time and alternating times respectively) and 7.9% Insulin glargine arms for whom data was missing at the time of the HbA1c measurement.
14.2 Type 1 Diabetes – Pediatric Patients 1 Year of Age and Older
Study J:Insulin Degludec Administered at the Same Time Each Day in Combination with a Rapid-Acting Insulin Analog at Mealtimes in Pediatric Patients 1 Year of Age and Older
The efficacy of Insulin Degludec was evaluated in a 26-week, randomized, open label, multicenter trial in 350 patients with type 1 diabetes mellitus (Study J). Patients were randomized to Insulin Degludec once-daily or insulin detemir once or twice-daily. Subjects on a twice-daily insulin detemir regimen were dosed at breakfast and in the evening either with the main evening meal or at bedtime. Insulin aspart was administered before each main meal in both treatment arms. At end of trial, 36% used insulin detemir once daily and 64% used insulin detemir twice daily.
The mean age of the trial population was 10 years; 24% were ages 1-5 years; 39% were ages 6-11 years and 36% were ages 12-17 years. The mean duration of diabetes was 4 years. 55% were male. 75% were White, 3% Black or African American. 3% were Hispanic. The mean z-score for body weight was 0.31.
At week 26, the difference in HbA1c reduction from baseline between Insulin Degludec and insulin detemir was 0.15% with a 95% confidence interval of [-0.03%; 0.33%] and met the pre-specified non-inferiority margin (0.4%). See Table 8.
Table 8: Results at Week 26 in a Trial Comparing Insulin Degludec to Insulin Detemir in Pediatric Patients 1 Year of Age and Older with Type 1 Diabetes Mellitus Receiving Insulin Aspart at Mealtimes
Insulin Degludec + Insulin aspart
Insulin detemir + Insulin aspart
N
174
176
HbA1c (%)
Baseline
8.2
8.0
End of 26 weeks
8.0
7.7
Adjusted mean change from baseline after 26 weeks ±
-0.19
-0.34
Estimated treatment difference [95%CI]
Insulin Degludec v. Insulin detemir
0.15 [ -0.03; 0.33]
FPG (mg/dL)
Baseline
162
151
End of 26 weeks
150
160
Adjusted mean change from baseline after 26 weeks
52.0
59.6
Daily basal insulin dose
Baseline mean
15 U (0.37 U/kg)
16 U (0.41 U/kg)
Mean dose after 26 weeks
16 U (0.37 U/kg)
22 U (0.51 U/kg)
Daily bolus insulin dose
Baseline mean
20 U (0.50 U/kg)
20 U (0.52 U/kg)
Mean dose after 26 weeks
23 U (0.56 U/kg)
22 U (0.57 U/kg)
- ±The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with missing data imputed by multiple imputation carrying forward the baseline value and adding the error term, with treatment, region, sex, and age group as fixed factors, and baseline HbA1c as covariate.
- In Study J, there were 2.9% of subjects in Insulin Degludec and 6.3% Insulin detemir arms for whom data was missing at the 26-week HbA1c measurement.
14.3 Type 2 Diabetes - Adult
Study D: Insulin Degludec Administered at the Same Time Each Day as an Add-on to Metformin with or without a DPP-4 Inhibitor in Insulin Naïve Adult Patients
The efficacy of Insulin Degludec was evaluated in a 52-week randomized, open-label, multicenter trial that enrolled 1030 insulin naïve patients with type 2 diabetes mellitus inadequately controlled on one or more oral antidiabetic agents (OADs). Patients were randomized to Insulin Degludec once-daily with the evening meal or insulin glargine U-100 once-daily according to the approved labeling. Metformin alone (83%) or in combination with a DPP-4 inhibitor (18%) was used as background therapy in both treatment arms.
The mean age of the trial population was 59 years and mean duration of diabetes was 9 years. 62% were male. 88% were White, 7% Black or African American. 17% were Hispanic. 10% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 31.1 kg/m2.
At week 52, the difference in HbA1c reduction from baseline between Insulin Degludec and insulin glargine U-100 was 0.09% with a 95% confidence interval of [-0.04%; 0.22%] and met the pre-specified non-inferiority margin (0.4%); See Table 9.
Table 9: Results at Week 52 in a Trial Comparing Insulin Degludec to Insulin Glargine U-100in Adult Patients with Type 2 Diabetes Mellitus on OAD(s)*
Insulin Degludec
+ OAD(s)*
Insulin glargine U-100 + OAD(s)*
N
773
257
HbA1c (%)
Baseline
8.2
8.2
End of trial
7.1
7.0
Adjusted mean change from baseline**
-1.06
-1.15
Estimated treatment difference [95%CI]
Insulin Degludec - Insulin glargine U-100
0.09 [-0.04;0.22]
Proportion Achieving HbA1c < 7% at Trial End
51.7%
54.1%
FPG (mg/dL)
Baseline
174
174
End of trial
106
115
Adjusted mean change from baseline
-68.0
-60.2
Daily insulin dose
Baseline mean (starting dose)
10 U
10 U
Mean dose after 52 weeks
56 U
58 U
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study D, there were 20.6% of subjects in the Insulin Degludec and 22.2% Insulin glargine arms for whom data was missing at the time of the HbA1c measurement.
Study E: Insulin Degludec U-200 Administered at the Same Time Each Day as an Add-on to Metformin with or without a DPP-4 Inhibitor in Insulin Naïve Adult Patients
The efficacy of Insulin Degludec U-200 was evaluated in a 26-week randomized, open-label, multicenter trial in 457 insulin naïve patients with type 2 diabetes mellitus inadequately controlled on one or more oral antidiabetic agents (OADs) at baseline. Patients were randomized to Insulin Degludec U-200 once-daily with the evening meal or insulin glargine U-100 once-daily according to the approved labeling. Both treatment arms were receiving metformin alone (84%) or in combination with a DPP-4 inhibitor (16%) as background therapy.
The mean age of the trial population was 58 years and mean duration of diabetes was 8 years. 53% were male. 78% were White, 14% Black or African American. 8% were Hispanic. 8% of patients had eGFR <60 mL/min/1.73m2. The mean BMI was approximately 32.4 kg/m2.
At week 26, the difference in HbA1c reduction from baseline between Insulin Degludec U-200 and insulin glargine U-100 was 0.04% with a 95% confidence interval of [-0.11%; 0.19%] and met the pre-specified non-inferiority margin (0.4%). See Table 10.
Table 10: Results at Week 26 in a Trial Comparing Insulin Degludec U-200 to Insulin Glargine U-100 in Adult Patients with Type 2 Diabetes Mellitus on OAD(s)*
Insulin Degludec U-200 + Met ± DPP-4
Insulin glargine U-100 + Met ± DPP-4
N
228
229
HbA1c (%)
Baseline
8.3
8.2
End of trial
7.0
6.9
Adjusted mean change from baseline**
-1.18
-1.22
Estimated treatment difference [95%CI]
Insulin Degludec - Insulin glargine U-100
0.04 [-0.11;0.19]
Proportion Achieving HbA1c < 7% at Trial End
52.2%
55.9%
FPG (mg/dL)
Baseline
172
174
End of trial
106
113
Adjusted mean change from baseline
-71.1
-63.5
Daily insulin dose
Baseline mean
10 U
10 U
Mean dose after 26 weeks
59 U
62 U
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study E, there were 12.3% of subjects in the Insulin Degludec and 12.7% Insulin glargine arms for whom data was missing at the time of the HbA1c measurement.
Study F: Insulin Degludec Administered at the Same Time Each Day in Insulin Naïve Adult Patients as an Add-on to One or More of the Following Oral Agents: Metformin, Sulfonylurea, Glinides or Alpha-Glucosidase Inhibitors
The efficacy of Insulin Degludec was evaluated in a 26-week randomized, open-label, multicenter trial in Asia in 435 insulin naïve patients with type 2 diabetes mellitus inadequately controlled on one or more oral antidiabetic agents (OADs) at baseline. Patients were randomized to Insulin Degludec once-daily in the evening or insulin glargine U-100 once-daily according to the approved labeling. Pre-trial oral antidiabetes agents were continued as background therapy except for DPP-4 inhibitors or thiazolidinediones in both treatment arms.
The mean age of the trial population was 59 years and mean duration of diabetes was 12 years. 54% were male. All patients were Asian. 11% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 25.0 kg/m2.
At week 26, the difference in HbA1c reduction from baseline between Insulin Degludec and insulin glargine U-100 was 0.11% with a 95% confidence interval of [-0.03%; 0.24%] and met the pre-specified non-inferiority margin (0.4%). See Table 11.
Table 11: Results at Week 26 in a Trial Comparing Insulin Degludec to Insulin Glargine U-100 in Adult Patients with Type 2 Diabetes Mellitus on OAD(s)*
Insulin Degludec + OAD(s)*
Insulin glargine U-100 + OAD(s)*
N
289
146
HbA1c (%)
Baseline
8.4
8.5
End of trial
7.2
7.1
Adjusted mean change from baseline**
-1.42
-1.52
Estimated treatment difference [95%CI] Insulin Degludec - Insulin glargine U-100
0.11 [-0.03 ; 0.24]
Proportion Achieving HbA1c < 7% at Trial End
40.8%
48.6%
FPG (mg/dL)
Baseline
152
156
End of trial
100
102
Adjusted mean change from baseline
-54.6
-53.0
Daily insulin dose
Baseline mean (starting dose)
9 U
9 U
Mean dose after 26 weeks
19 U
24 U
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study F, there were 10% of subjects in the Insulin Degludec and 6.8% Insulin glargine arms for whom data was missing at the time of the HbA1c measurement.
Study G: Insulin Degludec Administered at the Same Time Each Day or Any Time Each Day as an Add-on to One and up to Three of the Following Oral Agents: Metformin, Sulfonylurea or Glinides or Pioglitazone in Adult Patients
The efficacy of Insulin Degludec was evaluated in a 26-week randomized, open-label, multicenter trial in 687 patients with type 2 diabetes mellitus inadequately controlled on basal insulin alone, oral antidiabetic agents (OADs) alone or both basal insulin and OAD. Patients were randomized to Insulin Degludec injected once-daily at the same time each day (with the main evening meal), to Insulin Degludec injected once daily at any time each day or to insulin glargine U-100 injected once-daily according to the approved labeling. The any time each day Insulin Degludec arm was designed to simulate a worst-case scenario injection schedule of alternating short and long, once daily, dosing intervals (i.e., alternating intervals of 8 to 40 hours between doses). Insulin Degludec in this arm was dosed in the morning on Monday, Wednesday, and Friday and in the evening on Tuesday, Thursday, Saturday, and Sunday. Up to three of the following oral antidiabetes agents (metformin, sulfonylureas, glinides or thiazolidinediones) were administered as background therapy in both treatment arms.
The mean age of the trial population was 56 years and mean duration of diabetes was 11 years. 54% were male. 67% were White, 3% Black or African American. 11% were Hispanic. 6% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 29.6 kg/m2.
At week 26, the difference in HbA1c reduction from baseline between Insulin Degludec at alternating times and insulin glargine U-100 was 0.04% with a 95% confidence interval of [-0.12%; 0.20%]. This comparison met the pre-specified non-inferiority margin (0.4%). See Table 12.
Table 12: Results at Week 26 in a Trial Comparing Insulin Degludec at Same and Alternating Times to Insulin Glargine U-100 in Adult Patients with Type 2 Diabetes Mellitus on OAD(s)*
Insulin Degludec
at the same time each day ± OAD(s)*Insulin Degludec
at alternating times ± OAD(s)*Insulin glargine U-100 ± OAD(s)*
N
228
229
230
HbA1c (%)
Baseline
8.4
8.5
8.4
End of trial
7.3
7.2
7.1
Adjusted mean change from baseline**
-1.03
-1.17
-1.21
Estimated treatment difference [95%CI]
Insulin Degludec alternating- Insulin glargine U-100
0.04 [-0.12;0.20]
Estimated treatment difference Insulin Degludec alternating –Insulin Degludec same
-0.13
Proportion Achieving HbA1c < 7% at Trial End
40.8%
38.9%
43.9%
FPG (mg/dL)
Baseline
158
162
163
End of trial
105
105
112
Adjusted mean change from baseline
-54.2
-55.0
-47.5
Daily insulin dose
Baseline mean
21 U
19 U
19 U
Mean dose after 26 weeks
45 U
46 U
44 U
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study G, there were 11.4% subjects for Insulin Degludec (both same time and alternating times) and 11.7% Insulin glargine arms for whom data was missing at the time of the HbA1c measurement.
Study H: Insulin Degludec Administered at the Same Time Each Day in Combination with a Rapid-Acting Insulin Analog at Mealtimes in Adult Patients
The efficacy of Insulin Degludec was evaluated in a 52-week randomized, open-label, multicenter trial in 992 patients with type 2 diabetes mellitus inadequately controlled on premix insulin, bolus insulin alone, basal insulin alone, oral antidiabetic agents (OADs) alone or any combination thereof. Patients were randomized to Insulin Degludec once-daily with the main evening meal or insulin glargine U-100 once-daily according to the approved labeling. Insulin aspart was administered before each meal in both treatment arms. Up to two of the following oral antidiabetes agents (metformin or pioglitazone) were used as background therapy in both treatment arms.
The mean age of the trial population was 59 years and mean duration of diabetes was 14 years. 54% were male. 83% were White, 10% Black or African American. 12% were Hispanic. 12% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 32.2 kg/m2.
At week 52, the difference in HbA1c reduction from baseline between Insulin Degludec and insulin glargine U-100 was 0.08% with a 95% confidence interval of [-0.05%; 0.21%] and met the pre-specified non-inferiority margin (0.4%). See Table 13.
Table 13: Results at Week 52 in a Trial Comparing Insulin Degludec to Insulin Glargine U-100 in Adult Patients with Type 2 Diabetes Mellitus Receiving Insulin Aspart at Mealtimes and OADs*
Insulin Degludec + Insulin aspart ± OAD(s)*
Insulin glargine U-100 + Insulin aspart ± OAD(s)*
N
744
248
HbA1c (%)
Baseline
8.3
8.4
End of trial
7.1
7.1
Adjusted mean change from baseline**
-1.10
-1.18
Estimated treatment difference [95%CI]
Insulin Degludec - Insulin glargine U-100
0.08 [-0.05;0.21]
Proportion Achieving HbA1c < 7% at Trial End
49.5%
50.0%
FPG (mg/dL)
Baseline
166
166
End of trial
122
127
Adjusted mean change from baseline
-40.6
-35.3
Daily basal insulin dose
Baseline mean
42 U
41 U
Mean dose after 52 weeks
74 U
67 U
Daily bolus insulin dose
Baseline mean
33 U
33 U
Mean dose after 52 weeks
70 U
73 U
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study H, there were 16.1% of subjects in the Insulin Degludec and 14.5% Insulin glargine arms for whom data was missing at the time of the HbA1c measurement.
Study I: Insulin Degludec Administered at Any Time Each Day as an Add-on to One or Two of the Following Oral Agents: Metformin, Sulfonylurea, or Pioglitazone in Adult Patients
The efficacy of Insulin Degludec was evaluated in a 26-week randomized, open-label, multicenter trial in 447 patients with type 2 diabetes mellitus inadequately controlled on one or more oral antidiabetic agent (OADs) at baseline. Patients were randomized to Insulin Degludec once-daily at any time of day or sitagliptin once-daily according to the approved labeling. One or two of the following oral antidiabetes agents (metformin, sulfonylurea or pioglitazone) were also administered in both treatment arms.
The mean age of the trial population was 56 years and mean duration of diabetes was 8 years. 59% were male. 61% were White, 8% Black or African American. 21% were Hispanic. 6% of patients had eGFR<60 mL/min/1.73m2. The mean BMI was approximately 30.4 kg/m2.
At the end of 26 weeks, Insulin Degludec provided greater reduction in mean HbA1c compared to sitagliptin (p < 0.001). See Table 14.
Table 14: Results at Week 26 in a Trial Comparing Insulin Degludec to Sitagliptin in Adult Patients with Type 2 Diabetes Mellitus on OADs*
Insulin Degludec + OAD(s)*
Sitagliptin + OAD(s)*
N
225
222
HbA1c (%)
Baseline
8.8
9.0
End of trial
7.2
7.7
Adjusted mean change from baseline**
-1.52
-1.09
Estimated treatment difference [95%CI]
Insulin Degludec - Sitagliptin
-0.43 [-0.61;-0.24]1
Proportion Achieving HbA1c < 7% at Trial End
40.9%
27.9%
FPG (mg/dL)
Baseline
170
179
End of trial
112
154
Adjusted mean change from baseline
-61.4
-22.3
Daily insulin dose
Baseline mean
10 U
N/A
Mean dose after 26 weeks
43 U
N/A
- *OAD: oral antidiabetic agent
- **The change from baseline to end of treatment visit in HbA1c was analyzed using ANOVA with treatment, region, sex, and anti-diabetic treatment at screening as fixed effects, and age and baseline HbA1c as covariates.
- In Study I, there were 20.9% of subjects in the Insulin Degludec and 22.5% Sitagliptin arms for whom data was missing at the time of the HbA1c measurement.
- 1p <0.001; 1-sided p-value evaluated at 2.5% level for superiority
14.4 Safety Outcomes Trial
DEVOTE (NCT01959529) Cardiovascular Outcomes Trial of Insulin Degludec Administered Once-Daily Between Dinner and Bedtime in Combination with Standard of Care in Subjects with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease
DEVOTE was a multi-center, multi-national, randomized, double-blinded, active-controlled, treat-to-target, event-driven trial. 7,637 patients with inadequately controlled type 2 diabetes and atherosclerotic cardiovascular disease were randomized to either Insulin Degludec or insulin glargine U-100. Each was administered once-daily between dinner and bedtime in addition to standard of care for diabetes and cardiovascular disease for a median duration of 2 years.
Patients eligible to enter the trial were; 50 years of age or older and had established, stable, cardiovascular, cerebrovascular, peripheral artery disease, chronic kidney disease or NYHA class II and III heart failure (85% of the enrolled population) or were 60 years of age or older and had other specified risk factors for cardiovascular disease (15% of the enrolled population).
At baseline, demographic and disease characteristics were balanced between treatment groups. The mean age of the trial population was 65 years and the mean duration of diabetes was 16 years. The population was 63% male, 76% White 11% Black or African American, 10% Asian. 15% had Hispanic ethnicity. The mean HbA1c was 8.4% and the mean BMI was 33.6 kg/m2. The baseline mean estimated glomerular filtration rate (eGFR) was 68 mL/min/1.73m2. 41% of patients had eGFR 60-90 mL/min/1.73m2; 35% of patients had eGFR 30 to 60 mL/min/1.73 m2 and 3% of patients had eGFR <30 mL/min/1.73 m2. Previous history of severe hypoglycemia was not captured in the trial.
At baseline, patients treated their diabetes with oral antidiabetic drugs (72%) and with an insulin regimen (84%). Types of insulins included long acting insulin (60%), intermediate acting insulin (14%) short acting insulin (37%) and premixed insulin (10%). 16% of patients were insulin naive. The most common background oral antidiabetic drugs used at baseline were metformin (60%), sulfonylureas (29%) and DPP-4 inhibitors (12%).
During the trial, investigators could modify anti-diabetic and cardiovascular medications to achieve local standard of care treatment targets for lipids and blood pressure.
Cardiovascular Outcomes - Patients with T2DM and Atherosclerotic CVD
The incidence of major cardiovascular events with Insulin Degludec was evaluated in DEVOTE. Subjects treated with Insulin Degludec had a similar incidence of major adverse cardiovascular events (MACE) when compared to those treated with insulin glargine U-100.
The primary endpoint in DEVOTE was time from randomization to the first occurrence of a 3-component major adverse cardiovascular event (MACE): cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke. The trial was designed to exclude a pre-specified risk margin of 1.3 for the hazard ratio of MACE comparing Insulin Degludec to insulin glargine U-100. The primary outcome at end of trial was available for 98.2% of participants in each treatment group.
The time to first occurrence of MACE with Insulin Degludec as compared to insulin glargine U-100 was non-inferior (HR: 0.91; 95% CI [0.78;1.06]; see Figure 3). The results of the primary composite MACE endpoint and a summary of its individual components are shown in Table 15.
Table 15: Analysis of the Composite 3-point MACE and Individual Cardiovascular Endpoints in DEVOTE
Insulin Degludec
Insulin glargine U-100
N
3,818
3,819
Number of Patients (%)
Rate per 100 PYO*
Number of Patients (%)
Rate per 100 PYO*
Hazard Ratio
(95% CI)
Composite of first event of CV death, non-fatal MI, or non-fatal stroke (3-Point MACE)
325 (8.5)
4.41
356 (9.3)
4.86
0.91
[0.78; 1.06]
CV death
136 (3.6)
1.85
142 (3.7)
1.94
Non-fatal MI
144 (3.8)
1.95
169 (4.4)
2.31
Non-fatal stroke
71 (1.9)
0.96
79 (2.1)
1.08
* PYO = patient-years of observation until first MACE, death, or trial discontinuation
Figure 3: Cumulative Event Probability for Time to First MACE in DEVOTE
Hypoglycemia Outcomes - Patients with T2DM and Atherosclerotic CVD
The pre-specified secondary endpoints of event and incidence rates of severe hypoglycemia were sequentially tested.
Severe hypoglycemia was defined as an episode requiring assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions and during which plasma glucose concentration may not have been available, but where neurological recovery following the return of plasma glucose to normal was considered sufficient evidence that the event was induced by a low plasma glucose concentration.
The incidence of severe hypoglycemia was lower in the Insulin Degludec group as compared to the insulin glargine U-100 group (Table 16). Glycemic control between the two groups was similar at baseline and throughout the trial.
Table 16: Severe Hypoglycemic Episodes in Patients Treated with Insulin Degludec or Insulin Glargine U-100 in DEVOTE
Insulin Degludec
Insulin glargine U-100
N
3,818
3,819
Severe Hypoglycemia
Percent of patients with events
4.9%
6.6%
Estimated odds ratio [95%CI] Insulin Degludec/Insulin glargine U-100
0.73 [0.60; 0.89]*
Events per 100 Patient Years of Observation
3.70
6.25
Estimated rate ratio [95%CI] Insulin Degludec/Insulin glargine U-100
0.60 [0.48; 0.76]*
* Test for superiority evaluated at 5% level for significance, (2-sided p<0.001)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Insulin Degludec injection is available as a clear and colorless solution as follows:
Table 17: Presentations of Insulin Degludec
Insulin Degludec Presentation
Total volume
Concentration
Total units available in presentation
NDC number
Max dose per injection
Dose increment
Package Size
U-100
single-patient-use FlexTouch Pen
3 mL
100 units/mL
300 Units
73070-403-15
80 Units
1 Unit
5 pens/pack
U-100
multiple-dose
Vial
10 mL
100 units/mL
1,000 Units
73070-400-11
_
_
1 vial/pack
U-200
single-patient-use FlexTouch Pen
3 mL
200 units/mL
600 Units
73070-503-15
160 Units
2 Unit
3 pens/pack
Additional Information about Insulin Degludec FlexTouch:
- •
- Insulin Degludec U-100 FlexTouch pen dials in 1 unit increments.
- •
- Insulin Degludec U-200 FlexTouch pen dials in 2 unit increments.
16.2 Recommended Storage
Dispense in the original sealed carton with the enclosed Instructions for Use.
Store Insulin Degludec vials in the original carton to protect from light. Store unused Insulin Degludec in a refrigerator (36ºF to 46ºF [2ºC to 8ºC]). Do not store in the freezer or directly adjacent to the refrigerator cooling element. Do not freeze. Do not use Insulin Degludec if it has been frozen.
The storage conditions are summarized in Table 18:
Table 18: Storage Conditions for Insulin Degludec
Not in-use (unopened)
In-use (opened)
Refrigerated
(36°F to 46°F[2°C to 8°C])
Room Temperature
(up to 86°F [30°C])Room Temperature
(up to 86°F [30°C])Refrigerated
(36°F to 46°F[2°C to 8°C])
3 mL single-patient-use Insulin Degludec
U-100 FlexTouchUntil expiration date
56 days
(8 weeks)56 days
(8 weeks)
56 days
(8 weeks)
10 mL
multiple-dose Insulin Degludec
U-100 Vial
Until expiration date
56 days
(8 weeks)56 days
(8 weeks)56 days
(8 weeks)
3 mL single-patient-use Insulin Degludec
U-200 FlexTouchUntil expiration date
56 days
(8 weeks)56 days
(8 weeks)
56 days
(8 weeks)
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use). There are separate Instructions for Use for the Vials and FlexTouch Pens.
Never Share an Insulin Degludec FlexTouch Pen, Needle, or Insulin Syringe Between Patients
Advise patients that they should never share an Insulin Degludec FlexTouch pen device with another person, even if the needle is changed. Advise patients using Insulin Degludec vials not to share needles or insulin syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens [see Warnings and Precautions (5.1)].
Hyperglycemia or Hypoglycemia
Inform patients that hypoglycemia is the most common adverse reaction with insulin. Inform patients of the symptoms of hypoglycemia (e.g., impaired ability to concentrate and react). This may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery [see Warnings and Precautions (5.3)].
Advise patients that changes in insulin regimen can predispose to hyperglycemia or hypoglycemia and that changes in insulin regimen should be made under close medical supervision [see Warnings and Precautions (5.2)].
Hypoglycemia Due to Medication Errors
Inform patients to always check the insulin label before each injection to reduce the risk of a medication error [see Warnings and Precautions (5.4)]. Inform patients that the dose counter of Insulin Degludec FlexTouch pen shows the number of units of Insulin Degludec to be injected. NO dose re-calculation is required [see Dosage and Administration (2.2)]. Instruct patients to never use a syringe to remove Insulin Degludec from the FlexTouch disposable insulin prefilled pen.
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with Insulin Degludec. Inform patients on the symptoms of hypersensitivity reactions [see Warnings and Precautions (5.5)].
Version: 1
Novo Nordisk®, FlexTouch®, LEVEMIR®, NOVOLOG®, and NovoFine® are registered trademarks of Novo Nordisk A/S.
© 2022 Novo Nordisk
PATENT Information: http://novonordisk-us.com/products/product-patents.html
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
For information about Insulin Degludec contact:
Novo Nordisk Pharma, Inc.
1-800-727-6500
-
PATIENT INFORMATION
Insulin Degludec
injection, for subcutaneous use
This product is Tresiba (insulin degludec).
Do not share your Insulin Degludec FlexTouch insulin delivery device with other people, even if the needle has changed. Do not share needles or syringes with another person. You may give other people a serious infection, or get a serious infection from them.
What is Insulin Degludec?
- •
- Insulin Degludec is a man-made insulin that is used to control high blood sugar in adults and children who are 1 year of age and older with diabetes mellitus.
- •
- Insulin Degludec is not for people with diabetic ketoacidosis (increased ketones in the blood or urine).
- •
- It is not known if Insulin Degludec is safe and effective in children under 1 year of age.
- •
- Insulin Degludec is available in 2 concentrations (U-100 and U-200):
- o
- U-100 concentration is available in pen and vial
- o
- The 100 units/mL pen can be injected from 1 to 80 units in a single injection, in increments of 1 unit.
- o
- The 100 units/mL vial should be used with a U-100 insulin syringe only.
- o
- U-200 concentration is only available in pen
- o
- The 200 units/mL pen can be injected from 2 to 160 units in a single injection, in increments of 2 units.
Who should not take Insulin Degludec?
Do not take Insulin Degludec if you:
- •
- are having an episode of low blood sugar (hypoglycemia).
- •
- have an allergy to Insulin Degludec or any of the ingredients in Insulin Degludec.
Before taking Insulin Degludec, tell your healthcare provider about all your medical conditions including, if you are:
- •
- pregnant, planning to become pregnant, or are breastfeeding.
- •
- taking new prescription or over-the-counter medicines, vitamins, or herbal supplements.
Before you start taking Insulin Degludec, talk to your healthcare provider about low blood sugar and how to manage it.
How should I take Insulin Degludec?
- •
- Read the Instructions for Use that come with your Insulin Degludec.
- •
- Take Insulin Degludec exactly as your healthcare provider tells you to.
- •
- Do not do any conversion of your dose. The dose counter always shows the selected dose in units. Both the 100 units/mL and 200 units/mL Insulin Degludec FlexTouch pens are made to deliver your insulin dose in units.
- •
- Know the type and strength of insulin you take. Do not change the type of insulin you take unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you take different types of insulin.
- •
- For children who need less than 5 units of Insulin Degludec each day, use a Insulin Degludec U-100 vial.
- •
- Adults: If you miss or are delayed in taking your dose of Insulin Degludec:
- o
- Take your dose as soon as you remember then continue with your regular dosing schedule.
- o
- Make sure there are at least 8 hours between your doses.
- •
- If children miss a dose of Insulin Degludec:
- o
- Call the healthcare provider for information and instructions about checking blood sugar levels more often until the next scheduled dose of Insulin Degludec.
- •
- Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
- •
- Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
- •
- Never inject Insulin Degludec into a vein or muscle.
- •
- Never use a syringe to remove Insulin Degludec from the FlexTouch pen.
- •
- Insulin Degludec can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- •
-
Change (rotate) your injection sites within the area you choose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- o
- Do not use the exact same spot for each injection.
- o
- Do not inject where the skin has pits, is thickened, or has lumps.
- o
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
What should I avoid while taking Insulin Degludec?
While taking Insulin Degludec do not:
- •
- Drive or operate heavy machinery, until you know how Insulin Degludec affects you.
- •
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of Insulin Degludec?
Insulin Degludec may cause serious side effects that can lead to death, including:
- •
- Low blood sugar (hypoglycemia). Signs and symptoms that may indicate low blood sugar include:
- o
- dizziness or light-headedness
- o
- blurred vision
- o
- anxiety, irritability, or mood changes
- o
- sweating
- o
- slurred speech
- o
- hunger
- o
- confusion
- o
- shakiness
- o
- headache
- o
- fast heartbeat
- •
- Low potassium in your blood (hypokalemia).
- •
- Heart failure. Taking certain diabetes pills called thiazolidinediones or “TZDs” with Insulin Degludec may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure, it may get worse while you take TZDs with Insulin Degludec. Your healthcare provider should monitor you closely while you are taking TZDs with Insulin Degludec. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, tiredness, swelling of your ankles or feet and sudden weight gain. Treatment with TZDs and Insulin Degludec may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Your insulin dose may need to change because of:
- •
- change in level of physical activity or exercise
- •
- weight gain or loss
- •
- increased stress
- •
- illness
- •
- change in diet
Common side effects of Insulin Degludec may include:
- •
- serious allergic reactions (whole body reactions), reactions at the injection site, skin thickening or pits at the injection site (lipodystrophy), itching, rash, swelling of your hands and feet, and weight gain.
Get emergency medical help if you have:
- •
- trouble breathing, shortness of breath, fast heartbeat, swelling of your face, tongue, or throat, sweating, extreme drowsiness, dizziness, confusion.
These are not all the possible side effects of Insulin Degludec. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Insulin Degludec.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about Insulin Degludec that is written for health professionals. Do not use Insulin Degludec for a condition for which it was not prescribed. Do not give Insulin Degludec to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in Insulin Degludec?
Active Ingredient: insulin degludec
Inactive Ingredients: glycerin, metacresol, phenol, zinc, and Water for Injection, USP. Hydrochloric acid or sodium hydroxide may be added.
Manufactured by:
Novo Nordisk Inc., Plainsboro, NJ 08536, U.S. License Number 1261For more information call 1-800-727-6500.
This Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 07/2022
INSTRUCTIONS FOR USE
-
INSTRUCTIONS FOR USE
Insulin Degludec
injection, for subcutaneous use
10 mL multiple-dose vial (100 units/mL, U-100)
This product is Tresiba (insulin degludec).
Read this Instructions for Use before you start taking Insulin Degludec and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
The vial is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product and insulin syringe.
Do not reuse or share syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
Supplies you will need to give your Insulin Degludec injection:
- •
- a 10 mL Insulin Degludec vial
- •
- a U-100 insulin syringe and needle
- •
- 2 alcohol swabs
- •
- 1 sharps container for throwing away used syringes and needles. See “Disposing of your used needles and syringes” at the end of these instructions.
Preparing your Insulin Degludec dose:
- •
- Do not roll or shake the Insulin Degludec vial. Shaking the Insulin Degludec vial right before the dose is drawn into the syringe may cause bubbles or foam. This can cause you to draw up the wrong dose of insulin.
- •
- The tamper-resistant cap should not be loose or damaged before the first use. Do not use if the tamper-resistant cap is loose or damaged before using Insulin Degludec for the first time.
- •
- Wash your hands with soap and water.
- •
- Before you start to prepare your injection, check the Insulin Degludec label to make sure that you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- •
- Check that the Insulin Degludec vial is not cracked or damaged. Do not use if the Insulin Degludec vial is cracked or damaged.
- •
- Insulin Degludec should look clear and colorless. Do not use Insulin Degludec if it is thick, cloudy, or is colored.
- •
- Do not use Insulin Degludec past the expiration date printed on the label or 56 days after you start using the Insulin Degludec vial.
- Giving your Insulin Degludec injection:
- •
- Inject your Insulin Degludec exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- •
- Insulin Degludec can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen). Do not inject Insulin Degludec into your muscle.
- •
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting
- lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection
- sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has
- lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- •
- Do not dilute or mix Insulin Degludec with any other type of insulin or solutions.
After your injection:
- •
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
Disposing of your used needles and syringes:
Put your used insulin needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
-
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store Insulin Degludec?
Before use:
- •
- Store unopened Insulin Degludec vials in the refrigerator at 36°F to 46°F (2°C to 8°C) in the original carton to protect from light.
- •
- Do not freeze Insulin Degludec. Do not use Insulin Degludec if it has been frozen.
- •
- Unused Insulin Degludec vials may be used until the expiration date printed on the label, if they are kept in the refrigerator.
- •
- After 56 days, throw away Insulin Degludec vials that have been kept at room temperature (up to 86°F (30°C)).
Vial in use:
- •
- Store the Insulin Degludec vial you are currently using in the refrigerator between 36°F to 46°F (2°C to 8°C) or keep at room temperature up to 86°F (30°C) in the original carton to protect from light.
- •
- Keep Insulin Degludec away from direct heat or light.
- •
- The Insulin Degludec vial you are using should be thrown away after 56 days, even if it still has insulin left in it and the expiration date has not passed.
General information about the safe and effective use of Insulin Degludec
- •
- Keep Insulin Degludec vials, syringes, and needles out of the reach of children.
- •
- Always use a new syringe and needle for each injection to help ensure sterility and prevent blocked needles.
- •
- Do not reuse or share syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
Novo Nordisk® is a registered trademark of Novo Nordisk A/S.
Patent Information:
http://novonordisk-us.com/products/product-patents.html
Revised: 07/2022
© 2022 Novo Nordisk
For information contact:
- Novo Nordisk Pharma, Inc.
1-800-727-6500
(Se habla español)
Read before first use
-
INSTRUCTIONS FOR USE
Insulin Degludec
injection, for subcutaneous use
FlexTouch® Pen 100 units/mL
This product is Tresiba (insulin degludec).
Please read the following instructions carefully before using your Insulin Degludec FlexTouch Pen.
- •
- Do not share your Insulin Degludec FlexTouch Pen with other people, even if the needle is changed. You may give other people a serious infection, or get a serious infection from them.
- •
- Insulin Degludec FlexTouch Pen 100 units/mL (“Pen”) is a prefilled disposable, single-patient-use insulin pen containing 300 units of insulin degludec. You can inject from 1 to 80 units in a single injection. The units can be increased by 1 unit at a time.
- •
- This Pen is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product.
Supplies you will need to give your Insulin Degludec injection:
- •
- Insulin Degludec FlexTouch Pen
- •
- a new NovoFine or NovoTwist needle
- •
- alcohol swab
- •
- a sharps container for throwing away used Pens and needles. See “After your injection” at the end of these instructions.
Preparing your Insulin Degludec FlexTouch Pen:
- •
- Wash your hands with soap and water.
- •
- Before you start to prepare your injection, check the Insulin Degludec FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
- •
- Insulin Degludec should look clear and colorless. Do not use Insulin Degludec if it is cloudy or colored.
- •
- Do not use Insulin Degludec past the expiration date printed on the label or 56 days after you start using the Pen.
- •
- Always use a new needle for each injection to help ensure sterility and prevent blocked needles. Do not reuse or share needles with another person. You may give other people a serious infection, or get a serious infection from them.
Step 1:
- •
- Pull Pen cap straight off (See Figure B).
Step 2:
- •
- Check the liquid in the Pen (See Figure C). Insulin Degludec should look clear and colorless. Do not use it if it looks cloudy or colored.
Step 3:
- •
- Select a new needle.
- •
- Pull off the paper tab from the outer needle cap (See Figure D).
Step 4:
- •
- Push the capped needle straight onto the Pen and twist the needle on until it is tight (See Figure E).
Step 5:
- •
- Pull off the outer needle cap. Do not throw it away (See Figure F).
Step 6:
- •
- Pull off the inner needle cap and throw it away (See Figure G).
Priming your Insulin Degludec FlexTouch Pen:
Step 7:
- •
- Turn the dose selector to select 2 units (See Figure H).
Step 8:
- •
- Hold the Pen with the needle pointing up. Tap the top of the Pen gently a few times to let any air bubbles rise to the top (See Figure I).
Step 9:
- •
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter shows “0”. The “0” must line up with the dose pointer.
- •
- A drop of insulin should be seen at the needle tip (See Figure J).
- o
- If you do not see a drop of insulin, repeat steps 7 to 9, no more than 6 times.
- o
- If you still do not see a drop of insulin, change the needle and repeat steps 7 to 9.
Selecting your dose:
Step 10:
Insulin Degludec FlexTouch Pen 100 units/mL is made to deliver the number of insulin units that your healthcare provider prescribed. Do not perform any dose conversion.
Check to make sure the dose selector is set at 0.
- •
- Turn the dose selector to select the number of units you need to inject. The dose pointer should line up
with your dose (See Figure K).
- o
- If you select the wrong dose, you can turn the dose selector forwards or backwards to the correct dose.
- o
- The even numbers are printed on the dial.
- o
- The odd numbers are shown as lines.
- •
- The Insulin Degludec FlexTouch Pen insulin scale will show you how much insulin is left in your Pen (See Figure L).
- •
- To see how much insulin is left in your Insulin Degludec FlexTouch Pen:
- o
- Turn the dose selector until it stops. The dose counter will line up with the number of units of insulin that is left in your Pen. If the dose counter shows 80, there are at least 80 units left in your Pen.
- o
- If the dose counter shows less than 80, the number shown in the dose counter is the number of units left in your Pen.
Giving your injection:
- •
- Inject your Insulin Degludec exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- •
- Insulin Degludec can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- •
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
Step 11:
- •
- Choose your injection site (thighs, upper arms, or abdomen) and wipe the skin with an alcohol swab (See Figure M). Let the injection site dry before you inject your dose.
Step 12:
- •
- Insert the needle into your skin (See Figure N).
- o
- Make sure you can see the dose counter. Do not cover it with your fingers, this can stop your injection.
Step 13:
- •
- Press and hold down the dose button until the dose counter shows “0” (See Figure O).
- o
- The “0” must line up with the dose pointer. You may then hear or feel a click.
- •
- Keep the needle in your skin after the dose counter has returned to “0” and slowly count to 6 (See Figure P).
- o
- When the dose counter returns to “0”, you will not get your full dose until 6 seconds later.
- o
- If the needle is removed before you count to 6, you may see a stream of insulin coming from the needle tip.
- o
- If you see a stream of insulin coming from the needle tip you will not get your full dose. If this happens you should check your blood sugar levels more often because you may need more insulin.
Step 14:
- •
- Pull the needle out of your skin (See Figure Q).
- o
- If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol swab. Do not rub the area.
Step 15:
- •
- Carefully remove the needle from the Pen and throw it away (See Figure R).
- o
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
- •
- If you do not have a sharps container, carefully slip the needle into the outer needle cap (See Figure S). Safely remove the needle and throw it away as soon as you can.
- •
- Do not store the Pen with the needle attached. Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the Pen.
Step 16:
- •
- Replace the Pen cap by pushing it straight on (See Figure T).
After your injection:
- •
- The used Insulin Degludec FlexTouch Pen may be thrown away in your household trash after you have removed the needle.
- •
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my Insulin Degludec FlexTouch Pen?
Before use:
- •
- Store unused Insulin Degludec FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Do not freeze Insulin Degludec. Do not use Insulin Degludec if it has been frozen.
- •
- Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
Pen in use:
- •
- Store the Pen you are currently using in the refrigerator between 36°F to 46°F (2°C to 8°C) or keep at room temperature up to 86°F (30°C).
- •
- Keep Insulin Degludec away from heat or light.
- •
- The Insulin Degludec FlexTouch Pen you are using should be thrown away after 56 days if it is refrigerated or kept at room temperature, even if it still has insulin left in it and the expiration date has not passed.
General Information about the safe and effective use of Insulin Degludec.
- •
- Keep Insulin Degludec FlexTouch Pens and needles out of the reach of children.
- •
- Always use a new needle for each injection.
- •
- Do not share Insulin Degludec FlexTouch Pens or needles with other people. You may give other people a serious infection, or get a serious infection from them.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
For information contact:
Novo Nordisk Pharma, Inc.
1-800-727-6500 (Se habla español)
Revised: 07/2022
© 2022 Novo Nordisk
-
INSTRUCTIONS FOR USE
Insulin Degludec
injection, for subcutaneous use
FlexTouch® Pen 200 units/mL
This product is Tresiba (insulin degludec).
Please read the following instructions carefully before using your Insulin Degludec FlexTouch Pen.
- •
- Do not share your Insulin Degludec FlexTouch Pen with other people, even if the needle is changed. You may give other people a serious infection, or get a serious infection from them.
- •
- Insulin Degludec FlexTouch Pen 200 units/mL (“Pen”) is a prefilled disposable, single-patient-use insulin pen containing 600 units of insulin degludec. You can inject from 2 to 160 units in a single injection. The units can be increased by 2 units at a time.
- •
- Do not use a syringe to remove insulin from your Pen. If you do, you will get too many units of insulin because the scale on most syringes is for measuring U-100 insulin doses only.
- •
- This Pen is not recommended for use by the blind or visually impaired without the assistance of a person trained in the proper use of the product.
Supplies you will need to give your Insulin Degludec injection:
- •
- Insulin Degludec FlexTouch Pen
- •
- a new NovoFine or NovoTwist needle
- •
- alcohol swab
- •
- a sharps container for throwing away used Pens and needles. See “After your injection” at the end of these instructions.
Preparing your Insulin Degludec FlexTouch Pen:
- •
- Wash your hands with soap and water.
- •
- Before you start to prepare your injection, check the Insulin Degludec FlexTouch Pen label to make sure you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin.
- •
- Insulin Degludec should look clear and colorless. Do not use Insulin Degludec if it is cloudy or colored.
- •
- Do not use Insulin Degludec past the expiration date printed on the label or 56 days after you start using the Pen.
- •
- Always use a new needle for each injection to help ensure sterility and prevent blocked needles. Do not reuse or share needles with another person. You may give other people a serious infection, or get a serious infection from them.
(Figure A)
Step 1:
- •
- Pull Pen cap straight off (See Figure B).
Step 2:
- •
- Check the liquid in the Pen (See Figure C). Insulin Degludec should look clear and colorless. Do not use it if it looks cloudy or colored.
Step 3:
- •
- Select a new needle.
- •
- Pull off the paper tab from the outer needle cap (See Figure D).
Step 4:
- •
- Push the capped needle straight onto the Pen and twist the needle on until it is tight (See Figure E).
Step 5:
- •
- Pull off the outer needle cap. Do not throw it away (See Figure F).
Step 6:
- •
- Pull off the inner needle cap and throw it away (See Figure G).
Priming your Insulin Degludec FlexTouch Pen:
Step 7:
- •
- Turn the dose selector to select 2 units (See Figure H).
Step 8:
- •
- Hold the Pen with the needle pointing up. Tap the top of the Pen gently a few times to let any air bubbles rise to the top (See Figure I).
Step 9:
- •
- Hold the Pen with the needle pointing up. Press and hold in the dose button until the dose counter shows “0”. The “0” must line up with the dose pointer.
- •
- A drop of insulin should be seen at the needle tip (See Figure J).
- o
- If you do not see a drop of insulin, repeat steps 7 to 9, no more than 6 times.
- o
- If you still do not see a drop of insulin, change the needle and repeat steps 7 to 9.
Selecting your dose:
Step 10:
Insulin Degludec FlexTouch Pen 200 units/mL is made to deliver the number of insulin units that your healthcare provider prescribed. Do not perform any dose conversion.
Check to make sure the dose selector is set at 0.
- •
- Turn the dose selector to select the number of units you need to inject. The dose pointer should line up
with your dose (See Figure K).
- o
- If you select the wrong dose, you can turn the dose selector forwards or backwards to the correct dose.
- o
- Each line on the dial is an even number.
- •
- The Insulin Degludec FlexTouch Pen insulin scale will show you how much insulin is left in your Pen (See Figure L).
- •
- To see how much insulin is left in your Insulin Degludec FlexTouch Pen:
- o
- Turn the dose selector until it stops. The dose counter will line up with the number of units of insulin that is
left in your Pen. If the dose counter shows 160, there are at least 160 units left in your Pen.
- o
- If the dose counter shows less than 160, the number shown in the dose counter is the number of units left in your Pen.
Giving your injection:
- •
- Inject your Insulin Degludec exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- •
- Insulin Degludec can be injected under the skin (subcutaneously) of your upper legs (thighs), upper arms, or stomach area (abdomen).
- •
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
Step 11:
- •
- Choose your injection site (thighs, upper arms, or abdomen) and wipe the skin with an alcohol swab (See Figure M). Let the injection site dry before you inject your dose.
Step 12:
- •
- Insert the needle into your skin (See Figure N).
- o
- Make sure you can see the dose counter. Do not cover it with your fingers, this can stop your injection.
Step 13:
- •
- Press and hold down the dose button until the dose counter shows “0” (See Figure O).
- o
- The “0” must line up with the dose pointer. You may then hear or feel a click.
- •
- Keep the needle in your skin after the dose counter has returned to “0” and slowly count to 6 (See Figure P).
- o
- When the dose counter returns to “0”, you will not get your full dose until 6 seconds later.
- o
- If the needle is removed before you count to 6, you may see a stream of insulin coming from the needle tip.
- o
- If you see a stream of insulin coming from the needle tip you will not get your full dose. If this happens you should check your blood sugar levels more often because you may need more insulin.
Step 14:
- •
- Pullthe needleout of your skin (See Figure Q).
- o
- If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol swab. Do not rub the area.
Step 15:
- •
- Carefully remove the needle from the Pen and throw it away (See Figure R).
- o
- Do not recap the needle. Recapping the needle can lead to needle stick injury.
- o
- If you do not have a sharps container, carefully slip the needle into the outer needle cap (See Figure S). Safely remove the needle and throw it away as soon as you can.
- o
- Do not store the Pen with the needle attached. Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the Pen.
Step 16:
- o
- Replace the Pen cap by pushing it straight on (See Figure T).
After your injection:
- •
- The used Insulin Degludec FlexTouch Pen may be thrown away in your household trash after you have removed the needle.
- •
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- •
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- o
- made of a heavy-duty plastic
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- o
- upright and stable during use
- o
- leak-resistant
- o
- properly labeled to warn of hazardous waste inside the container
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share needles or syringes with another person. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store my Insulin Degludec FlexTouch Pen?
Before use:
- •
- Store unused Insulin Degludec FlexTouch Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Do not freeze Insulin Degludec. Do not use Insulin Degludec if it has been frozen.
- •
- Unused Pens may be used until the expiration date printed on the label, if kept in the refrigerator.
Pen in use:
- •
- Store the Pen you are currently using in the refrigerator between 36°F to 46°F (2°C to 8°C) or keep at room temperature up to 86°F (30°C).
- •
- Keep Insulin Degludec away from heat or light.
- •
- The Insulin Degludec FlexTouch Pen you are using should be thrown away after 56 days if it is refrigerated or kept at room temperature, even if it still has insulin left in it and the expiration date has not passed.
General Information about the safe and effective use of Insulin Degludec.
- •
- Keep Insulin Degludec FlexTouch Pens and needles out of the reach of children.
- •
- Always use a new needle for each injection.
- •
- Do not share Insulin Degludec FlexTouch Pens or needles with other people. You may give other people a serious infection, or get a serious infection from them.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
For information contact:
Novo Nordisk Pharma, Inc.
1-800-727-6500 (Se habla español)Revised: 07/2022
© 2022 Novo Nordisk
- PRINCIPAL DISPLAY PANEL U-100 Vial
-
PRINCIPAL DISPLAY PANEL U-100 FlexTouch
NDC 73070-403-15 List: 040315
Insulin Degludec
Injection
FlexTouch®
For Single Patient Use Only
100 units/mL (U-100)
This product is Tresiba (insulin degludec).
5×3 mL Prefilled Pens
For subcutaneous use only
Recommended for use with NovoFine®,
NovoFine® Plus or NovoTwist® disposable needles.Keep in a cold place until first use.
Store at 36°F to 46°F (2°C to 8°C).
Do not freeze.
After first use, store between 36°F to 86°F (2°C to 30°C) for up to 56 days.
Protect from light.
Rx Only
Dispense in this sealed carton.
-
PRINCIPAL DISPLAY PANEL U-200 FlexTouch
- NDC 73070-503-15List: 050315
Insulin Degludec
Injection
FlexTouch®
For Single Patient Use Only
200 units/mL (U-200)
This product is Tresiba (insulin degludec).
3×3 mL Prefilled Pens
For subcutaneous use only
Recommended for use with
NovoFine®, NovoFine® Plus or NovoTwist® disposable needles.
disposable needles.Keep in a cold place until first use.
Store at 36°F to 46°F (2°C to 8°C).
Do not freeze.
After first use, store between 36°F to 86°F (2°C to 30°C) for up to 56 days.
Protect from light.
Rx Only
Dispense in this sealed carton.
-
INGREDIENTS AND APPEARANCE
INSULIN DEGLUDEC
insulin degludec injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73070-403 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN DEGLUDEC (UNII: 54Q18076QB) (INSULIN DEGLUDEC - UNII:54Q18076QB) INSULIN DEGLUDEC 100 U in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 19.6 mg in 1 mL PHENOL (UNII: 339NCG44TV) 1.50 mg in 1 mL METACRESOL (UNII: GGO4Y809LO) 1.72 mg in 1 mL ZINC (UNII: J41CSQ7QDS) 32.7 ug in 1 mL WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73070-403-15 5 in 1 CARTON 07/01/2022 1 3 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA203314 07/01/2022 INSULIN DEGLUDEC
insulin degludec injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73070-503 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN DEGLUDEC (UNII: 54Q18076QB) (INSULIN DEGLUDEC - UNII:54Q18076QB) INSULIN DEGLUDEC 200 U in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 19.6 mg in 1 mL PHENOL (UNII: 339NCG44TV) 1.50 mg in 1 mL METACRESOL (UNII: GGO4Y809LO) 1.72 mg in 1 mL ZINC (UNII: J41CSQ7QDS) 71.9 ug in 1 mL WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73070-503-15 3 in 1 CARTON 07/01/2022 1 3 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA203314 07/01/2022 INSULIN DEGLUDEC
insulin degludec injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73070-400 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN DEGLUDEC (UNII: 54Q18076QB) (INSULIN DEGLUDEC - UNII:54Q18076QB) INSULIN DEGLUDEC 100 U in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) 19.6 mg in 1 mL PHENOL (UNII: 339NCG44TV) 1.50 mg in 1 mL METACRESOL (UNII: GGO4Y809LO) 1.72 mg in 1 mL ZINC (UNII: J41CSQ7QDS) 32.7 ug in 1 mL WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73070-400-11 1 in 1 CARTON 07/01/2022 1 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA203314 07/01/2022 Labeler - Novo Nordisk Pharma, Inc. (117032563) Establishment Name Address ID/FEI Business Operations Novo Nordisk A/S 305914798 MANUFACTURE(73070-403, 73070-503, 73070-400) Establishment Name Address ID/FEI Business Operations Novo Nordisk A/S 305156788 API MANUFACTURE(73070-403, 73070-503, 73070-400) Establishment Name Address ID/FEI Business Operations Novo Nordisk Pharmaceutical Industries, LP 622920320 MANUFACTURE(73070-403, 73070-503, 73070-400)