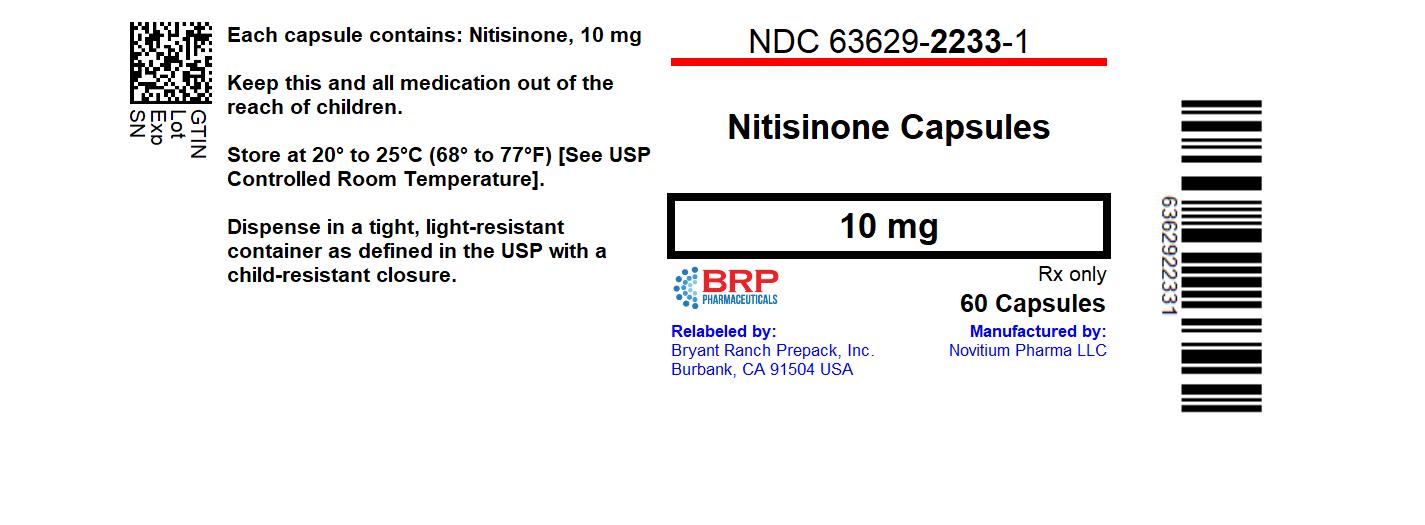
Label: NITISINONE capsule
- NDC Code(s): 63629-2233-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 0254-3022
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated January 10, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NITISINONE CAPSULES safely and effectively. See full prescribing information for NITISINONE CAPSULES.
NITISINONE capsules, for oral use.
Initial U.S. Approval: 2002
RECENT MAJOR CHANGES
Warnings and Precautions (5.1) 05/2019
INDICATIONS AND USAGE
Nitisinone is a hydroxy-phenylpyruvate dioxygenase inhibitor indicated for the treatment of adult and pediatric patients with hereditary tyrosinemia type 1 (HT-1) in combination with dietary restriction of tyrosine and phenylalanine. (1)
DOSAGE AND ADMINISTRATION
Recommended Dosage (2.1):
- The recommended starting dosage is 0.5 mg/kg orally twice daily.
- Titrate the dosage based on biochemical and/or clinical response, as described in the full prescribing information.
- The maximum total daily dosage is 2 mg/kg orally.
Administration (2.2):
- Maintain dietary restriction of tyrosine and phenylalanine
- Take Nitisinone capsules at least one hour before, or two hours after a meal
- For patients who have difficulties swallowing capsules, the capsules may be opened and the contents suspended in a small amount of water, formula or apple sauce immediately before use.
DOSAGE FORMS AND STRENGTHS
Capsules: 2 mg, 5 mg, 10 mg. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Elevated Plasma Tyrosine Levels, Ocular Symptoms, Developmental Delay and Hyperkeratotic Plaques: Inadequate restriction of tyrosine and phenylalanine intake can lead to elevations in plasma tyrosine, which at levels above 500 micromol/L can result in symptoms, intellectual disability and developmental delay or painful hyperkeratotic plaques on the soles and palms; do not adjust nitisinone dosage in order to lower the plasma tyrosine concentration. Obtain slit-lamp examination prior to treatment, regularly during treatment; Reexamine patients if symptoms develop or tyrosine levels are > 500 micromol/L. Assess plasma tyrosine levels in patients with an abrupt change in neurologic status. (5.1)
- Leukopenia and Severe Thrombocytopenia: Monitor platelet and white blood cell counts. (5.2)
ADVERSE REACTIONS
Most common adverse reactions (>1%) are elevated tyrosine levels, thrombocytopenia, leukopenia, conjunctivitis, corneal opacity, keratitis, photophobia, eye pain, blepharitis, cataracts, granulocytopenia, epistaxis, pruritus, exfoliative dermatitis, dry skin, maculopapular rash and alopecia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novitium Pharma at 1-855-204-1431 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP2C9 Substrates: Increased systemic exposure of these co-administered drugs; reduce the dosage. Additional dosage adjustments may be needed to maintain therapeutic drug concentrations for narrow therapeutic index drugs. (7)
- OAT1/OAT3 Substrates: Increased systemic exposure of these co-administered drugs; monitor for potential adverse reactions. (7)
Additional pediatric use information is approved for Swedish Orphan Biovitrum AB PUBL's Orfadin (nitisinone) capsules. However, due to Swedish Orphan Biovitrum AB PUBL's marketing exclusivity rights, this drug product is not labeled with that pediatric information.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
2 DOSAGE & ADMINISTRATION
2.1 Dosage
2.2 Administration
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Elevated Plasma Tyrosine Levels, Ocular Symptoms, Developmental Delay and Hyperkeratotic Plaques
5.2 Leukopenia and Severe Thrombocytopenia
6 ADVERSE REACTIONS
6.1 Clinical trials experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELLING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS & USAGE
-
2 DOSAGE & ADMINISTRATION
2.1 Dosage
Starting Dosage
The recommended starting dosage of Nitisinone capsules is 0.5 mg/kg administered orally twice daily
Dosage Titration
Titrate the dosage in each individual patient based on biochemical and/or clinical response.
- Monitor plasma and/or urine succinylacetone concentrations, liver function parameters and alpha-fetoprotein levels.
- If succinylacetone is still detectable in blood or urine 4 weeks after the start of nitisinone treatment, increase the nitisinone dosage to 0.75 mg/kg twice daily. A maximum total daily dosage of 2 mg/kg may be needed based on the evaluation of all biochemical parameters.
- If the biochemical response is satisfactory (undetectable blood and/or urine succinylacetone), the dosage should be adjusted only according to body weight gain and not according to plasma tyrosine levels.
- During initiation of therapy, or if there is a deterioration in the patient’s condition, it may be necessary to follow all available biochemical parameters more closely (i.e. plasma and/or urine succinylacetone, urine 5-aminolevulinate (ALA) and erythrocyte porphobilinogen (PBG)-synthase activity).
- Maintain plasma tyrosine levels below 500 micromol/L by dietary restriction of tyrosine and phenylalanine intake [see Warnings and Precautions (5.1)]. In patients who develop plasma tyrosine levels above 500 micromol/L, assess dietary tyrosine and phenylalanine intake. Do not adjust the nitisinone dosage in order to lower the plasma tyrosine concentration.
Additional pediatric use information is approved for Swedish Orphan Biovitrum AB PUBL’s ORFADIN (nitisinone) capsules. However, due to Swedish Orphan Biovitrum AB PUBL’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.2 Administration
Administration of Nitisinone Capsules:
- Maintain dietary restriction of tyrosine and phenylalanine when taking nitisinone.
- Capsules:Take at least one hour before, or two hours after a meal [see Clinical Pharmacology (12.3)]. For patients who have difficulty swallowing the capsules, the capsules may be opened and the contents suspended in a small amount of water, formula or apple sauce immediately before use.
-
3 DOSAGE FORMS & STRENGTHS
Nitisinone Capsules are available as:
2 mg: White Opaque Hard gelatin capsule shell Size #3, imprinted with black ink as "008" on cap and "Novitium 2 mg" on body, filled with white to off white powder blend.
5 mg: White Opaque Hard gelatin capsule shell Size #3, imprinted with black ink as "009" on cap and "Novitium 5 mg" on body, filled with white to off white powder blend.
10 mg: White Opaque Hard gelatin capsule shell Size #3, imprinted with black ink as "010" on cap and "Novitium 10 mg" on body, filled with white to off white powder blend.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Elevated Plasma Tyrosine Levels, Ocular Symptoms, Developmental Delay and Hyperkeratotic Plaques
Nitisinone is an inhibitor of 4-hydroxyphenyl-pyruvate dioxygenase, an enzyme in the tyrosine metabolic pathway [see Clinical Pharmacology (12.1)]. Therefore, treatment with nitisinone may cause an increase in plasma tyrosine levels in patients with HT-1. Maintain concomitant reduction in dietary tyrosine and phenylalanine while on nitisinone treatment. Do not adjust nitisinone dosage in order to lower the plasma tyrosine concentration. Maintain plasma tyrosine levels below 500 micromol/L. Inadequate restriction of tyrosine and phenylalanine intake can lead to elevations in plasma tyrosine levels and levels greater than 500 micromol/L may lead to the following:
- Ocular signs and symptoms including corneal ulcers, corneal opacities, keratitis, conjunctivitis, eye pain, and photophobia have been reported in patients treated with nitisinone [see Adverse Reactions (6.1)]. In a clinical study in a non HT-1 population without dietary restriction and reported tyrosine levels >500 micromol/l both symptomatic and asymptomatic keratopathies have been observed. Therefore, perform a baseline ophthalmologic examination including slit-lamp examination prior to initiating nitisinone treatment and regularly thereafter. Patients who develop photophobia, eye pain, or signs of inflammation such as redness, swelling, or burning of the eyes or tyrosine levels are >500 micromol/L during treatment with nitisinone should undergo slit-lamp reexamination and immediate measurement of the plasma tyrosine concentration.
- Variable degrees of intellectual disability and developmental delay. In patients treated with nitisinone who exhibit an abrupt change in neurologic status, perform a clinical laboratory assessment including plasma tyrosine levels.
- Painful hyperkeratotic plaques on the soles and palms
In patients with HT-1 treated with dietary restrictions and nitisinone who develop elevated plasma tyrosine levels, assess dietary tyrosine and phenylalanine intake.
5.2 Leukopenia and Severe Thrombocytopenia
In clinical trials, patients treated with nitisinone and dietary restriction developed transient leukopenia (3%), thrombocytopenia (3%), or both (1.5%) [see Adverse Reactions (6.1)]. No patients developed infections or bleeding as a result of the episodes of leukopenia and thrombocytopenia. Monitor platelet and white blood cell counts during nitisinone therapy.
-
6 ADVERSE REACTIONS
6.1 Clinical trials experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Nitisinone was studied in one open-label, uncontrolled study of 207 patients with HT-1, ages 0 to 22 years at enrollment (median age 9 months), who were diagnosed with HT-1 by the presence of succinylacetone in the urine or plasma. The starting dose of nitisinone was 0.3 to 0.5 mg/kg twice daily, and the dose was increased in some patients to 1 mg/kg twice daily based on weight, biochemical, and enzyme markers. The recommended starting dosage of nitisinone is 0.5 mg/kg twice daily [see Dosage and Administration (2.1)]. Median duration of treatment was 22 months (range 0.1 to 80 months).
The most serious adverse reactions reported during nitisinone treatment were thrombocytopenia, leukopenia, porphyria, and ocular/visual complaints associated with elevated tyrosine levels [see Warnings and Precautions (5.1, 5.2)]. Fourteen patients experienced ocular/visual events. The duration of the symptoms varied from 5 days to 2 years. Six patients had thrombocytopenia, three of which had platelet counts 30,000/microL or lower. In 4 patients with thrombocytopenia, platelet counts gradually returned to normal (duration up to 47 days) without change in nitisinone dose. No patients developed infections or bleeding as a result of the episodes of leukopenia and thrombocytopenia.
Patients with HT- 1 are at increased risk of developing porphyric crises, hepatic neoplasms, and liver failure requiring liver transplantation. These complications of HT-1 were observed in patients treated with nitisinone for a median of 22 months during the clinical trial (liver transplantation 13%, liver failure 7%, malignant hepatic neoplasms 5%, benign hepatic neoplasms 3%, porphyria 1%).
The most common adverse reactions reported in the clinical trial are summarized in Table 1.
TABLE 1
Most Common Adverse Reactions in Patients with HT-1 Treated with Nitisinone*Elevated tyrosine levels >10% Leukopenia 3% Thrombocytopenia 3% Conjunctivitis 2% Corneal opacity 2% Keratitis 2% Photophobia 2% Eye pain 1% Blepharitis 1% Cataracts 1% Granulocytopenia 1% Epistaxis 1% Pruritus 1% Exfoliative dermatitis 1% Dry skin 1% Maculopapular rash 1% Alopecia 1% *reported in at least 1% of patients
Adverse reactions reported in less than 1% of the patients, included death, seizure, brain tumor, encephalopathy, hyperkinesia, cyanosis, abdominal pain, diarrhea, enanthema, gastrointestinal hemorrhage, melena, elevated hepatic enzymes, liver enlargement, hypoglycemia, septicemia, and bronchitis.
-
7 DRUG INTERACTIONS
Nitisinone is a moderate CYP2C9 inhibitor, a weak CYP2E1 inducer and an inhibitor of OAT1/OAT3. Table 2 includes drugs with clinically important drug interactions when administered concomitantly with nitisinone and instructions for preventing or managing them.
Table 2: Clinically Relevant Interactions Affecting Co-Administered Drugs
Sensitive CYP2C9 Substrates (e.g., celecoxib, tolbutamide) or CYP2C9 Substrates with a Narrow Therapeutic Index (e.g., phenytoin, warfarin)
Clinical Impact
Increased exposure of the co-administered drugs metabolized by CYP2C9. [see Clinical Pharmacology (12.3)]
Intervention
Reduce the dosage of the co-administered drugs metabolized by CYP2C9 drug by half. Additional dosage adjustments may be needed to maintain therapeutic drug concentrations for narrow therapeutic index drugs. See prescribing information for those drugs.
OAT1/OAT3 Substrates (e.g., adefovir, ganciclovir, methotrexate)
Clinical Impact
Increased exposure of the interacting drug [see Clinical Pharmacology (12.3)]
Intervention
Monitor for potential adverse reactions related to the co-administered drug.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited available data with nitisinone use in pregnant women are not sufficient to determine a drug-associated risk of adverse developmental outcomes. Animal reproduction studies have been conducted for nitisinone. In these studies, nitisinone was administered to mice and rabbits during organogenesis with oral doses of nitisinone up to 20 and 8 times respectively, the recommended initial dose of 1 mg/kg/day. In mice, nitisinone caused incomplete skeletal ossification of fetal bones and decreased pup survival at doses 0.4 times the recommended initial dose, and increased gestational length at doses 4 times the recommended initial dose. In rabbits, nitisinone caused maternal toxicity and incomplete skeletal ossification of fetal bones at doses 1.6 times the recommended initial dose [see Data].
The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Reproduction studies have been performed in mice at oral doses of about 0.4, 4 and 20 times the recommended initial dose (1 mg/kg/day) and in rabbits at oral doses of about 1.6, 4 and 8 times the recommended initial dose based on the body surface area. In mice, nitisinone has been shown to cause incomplete skeletal ossification of fetal bones at 0.4, 4 and 20 times the recommended initial dose, increased gestational length at 4 and 20 times the recommended initial dose, and decreased pup survival at 0.4 times the recommended initial dose based on the body surface area. In rabbits, nitisinone caused incomplete skeletal ossification of fetal bones at 1.6, 4 and 8 times the recommended initial dose based on the body surface area.
8.2 Lactation
Risk Summary
There are no data on the presence of nitisinone in human milk, the effects on the breastfed infant, or the effects on milk production. Data suggest that nitisinone is present in rat milk due to findings of ocular toxicity and lower body weight seen in drug naive nursing rat pups. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for nitisinone and any potential adverse effects on the breastfed infant from nitisinone or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of nitisinone have been established in pediatric patients for the treatment of HT-1 in combination with dietary restriction of tyrosine and phenylalanine. Use of nitisinone in pediatric patients is supported by evidence from one open-label, uncontrolled clinical study conducted in 207 patients with HT-1 ages 0 to 22 years (median age 9 months) [see Clinical Studies (14)].
8.5 Geriatric Use
Clinical studies of nitisinone did not include any subjects aged 65 and over. No pharmacokinetic studies of nitisinone have been performed in geriatric patients. In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy in this patient population.
-
10 OVERDOSAGE
Accidental ingestion of nitisinone by individuals eating normal diets not restricted in tyrosine and phenylalanine will result in elevated tyrosine levels. In healthy subjects given a single 1 mg/kg dose of nitisinone, the plasma tyrosine level reached a maximum of 1200 micromol/L at 48 to 120 hours after dosing. After a washout period of 14 days, the mean value of plasma tyrosine was still 808 micromol/L. Fasted follow-up samples obtained from volunteers several weeks later showed tyrosine values back to normal. There were no reports of changes in vital signs or laboratory data of any clinical significance. One patient reported sensitivity to sunlight. Hyper-tyrosinemia has been reported with nitisinone treatment [see Warnings and Precautions (5.1)].
-
11 DESCRIPTION
Nitisinone capsules contains nitisinone, which is a hydroxyphenyl-pyruvate dioxygenase inhibitor indicated as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of hereditary tyrosinemia type 1 (HT-1).
Nitisinone occurs as white to yellow colored powder. It is practically insoluble in water and sparingly soluble in 2M Sodium Hydroxide, Ethanol and Methanol.
Chemically, nitisinone is 2-[2-nitro-4-(trifluoromethyl)benzoyl] cyclohexane-1,3-dione, and the structural formula is:
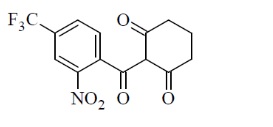
Figure 1. The molecular formula is C14H10F3NO5 with a relative mass of 329.23
Inert ingredients in the formulation are: Citric acid anhydrous, hypromellose, mannitol and stearic acid. The empty hard gelatin capsules contains gelatin, sodium lauryl sulfate and titanium dioxide.
The capsules shells are imprinted in edible ink which contains black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nitisinone is a competitive inhibitor of 4-hydroxyphenyl-pyruvate dioxygenase, an enzyme upstream of fumarylacetoacetate hydrolase (FAH) in the tyrosine catabolic pathway. By inhibiting the normal catabolism of tyrosine in patients with HT-1, nitisinone prevents the accumulation of the catabolic intermediates maleylacetoacetate and fumarylacetoacetate. In patients with HT-1, these catabolic intermediates are converted to the toxic metabolites succinylacetone and succinylacetoacetate, which are responsible for the observed liver and kidney toxicity. Succinylacetone can also inhibit the porphyrin synthesis pathway leading to the accumulation of 5-aminolevulinate, a neurotoxin responsible for the porphyric crises characteristic of HT-1.
12.2 Pharmacodynamics
In a clinical study, patients with HT-1 were diagnosed by the presence of succinylacetone in urine or plasma and treated with nitisinone [see Clinical Studies (14)]. In all 186 patients whose urine succinylacetone was measured, the urinary succinylacetone concentration decreased to less than 1 mmol/mol creatinine, the lower limit of quantitation. The median time to normalization of urine succinylacetone was 0.3 months. The probability of recurrence of abnormal values of urine succinylacetone was 1% at a nitisinone concentration of 37 micromol/L (95% confidence interval: 23, 51 micromol/L). In 87% (150/172) of patients whose plasma succinylacetone was measured, the plasma succinylacetone concentration decreased to less than 0.1 micromol/L, the lower limit of quantitation. The median time to normalization of plasma succinylacetone was 3.9 months.
Nitisinone inhibits catabolism of the amino acid tyrosine and can result in elevated plasma levels of tyrosine. Therefore, treatment with nitisinone requires restriction of the dietary intake of tyrosine and phenylalanine to prevent the toxicity associated with elevated plasma levels of tyrosine [see Warnings and Precautions (5.1)].
Additional pediatric use information is approved for Swedish Orphan Biovitrum ABPUBL's Orfadin (nitisinone) capsules. However, due to Swedish Orphan Biovitrum ABPUBL's marketing exclusivity rights, this drug product is not labeled with that pediatric information.12.3 Pharmacokinetics
The single-dose pharmacokinetics of nitisinone have been studied for nitisinone capsules in healthy adult subjects and the multiple-dose pharmacokinetics have been studied for nitisinone capsules in healthy subjects.
Absorption
The pharmacokinetic characteristics following single oral administration of nitisinone 30 mg under fasting conditions are shown in Table 3. The multiple-dose characteristics of nitisinone 80 mg once daily are shown in Table 4. Steady-state (SS) was reached within 14 days dosing in all subjects.
TABLE 3
Nitisinone Arithmetic Mean (CV%) Pharmacokinetic Parameters in Healthy Subjects Following a Single Oral 30 mg Dose of Nitisinone Under Fasting Conditions
Treatment
Cmax (micromol/L)
[range]
tmax* (h)
[range]
AUC0-72h
(micromol·h/L)
[range]
Nitisinone capsule (n=12)
10.5 (26)
3.5
[0.8 to 8.0]
406 (13)
* presented as median [range]
TABLE 4
Nitisinone Arithmetic Mean (CV%) Pharmacokinetic Parameters in Healthy Subjects Following Repeated Once Daily Administration of 80 mg Nitisisnone Under Fasting Conditions.
Cmax,ss (micromol/L)
Cmin,ss (micromol/L)
tmax,ss* (h)
AUC0-24h,ss
(micromol·h/L)
Treatment
[CV%]
[range]
[range]
[range]
Nitisisnone capsule
(n=18)
120 (23)
73(24)
4.0
2204(18)
[0.0 to 16.0]
* presented as median [range]
Food Effect: No food effect study was conducted with nitisinone capsules.
Distribution
In vitro binding of nitisinone to human plasma proteins is greater than 95% at 50 micromolar concentration.
Elimination
The mean terminal plasma half-life of single dose nitisinone in healthy male subjects is 54 hours.
The mean (CV%) apparent plasma clearance in 18 healthy adults following multiple once daily doses of Nitisinone 80 mg is 113 (16) mL/hr.
Metabolism: In vitro studies have shown that nitisinone is relatively stable in human liver microsomes with minor metabolism possibly mediated by CYP3A4 enzyme.Excretion: Renal elimination of nitisinone is of minor importance, since the mean of the fraction of dose excreted as unchanged nitisinone in the urine (fe(%)) was 3.0% (n=3) following multiple oral doses of 80 mg daily in healthy subjects. The estimated mean (CV%) renal clearance of nitisinone was 0.003 L/h (25%).
Drug Interaction Studies
Nitisinone does not inhibit CYP2D6. Nitisinone is a moderate inhibitor of CYP2C9, and a weak inducer of CYP2E1 (Table 5). Nitisinone is an inhibitor of OAT1/3 (Table 5)
Table 5. Percent Change in AUC0-∞ and Cmax for Co-administered Drugs in the Presence of Nitisinone in 18 Healthy Subjects
Co-administered
Drug a
Dose of
Co-administered Drug (Route of Administration)
Effect of Nitisinone on the Pharmacokinetics of Co-administered Drug b
AUC0-∞
Cmax
CYP2C9 Substrate Tolbutamide c
500 mg (oral)
131% ↑
16% ↑
CYP2E1 Substrate
Chlorzoxazone
250 mg (oral)
27% ↓
18% ↓
OAT1/3 Substrate
Furosemide
20 mg (intravenous)
72% ↑
12% ↑
↑ = Increased; ↓ = Decreased
a The interacting drug was administered alone on Day 1 and together with nitisinone on Day 17.
b Multiple doses of 80 mg nitisinone were administered daily alone from Day 3 to Day 16.
c 16 subjects in Period 2 received nitisinone and tolbutamide while 18 subjects in Period 1 received nitisinone alone.
In Vitro Studies Where Drug Interaction Potential Was Not Further Evaluated Clinically
In vitro studies showed that nitisinone does not inhibit CYP1A2, 2C19, or 3A4. Nitisinone does not induce CYP1A2, 2B6 or 3A4/5. Nitisinone does not inhibit P-gp, BCRP, OATP1B1, OATP1B3 and OCT2-mediated transports at therapeutically relevant concentrations. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment Of Fertility
The carcinogenic potential of nitisinone was assessed in a 26-week oral (gavage) carcinogenicity study in Tg.rasH2 mice. There were no drug-related neoplastic findings in male or female Tg.rasH2 mice at doses up to 100 mg/kg/ day nitisinone (approximately 8.1 times the recommended initial dose of 1 mg/kg/day on a body surface area basis).
Nitisinone was not genotoxic in the Ames test and the in vivo mouse liver unscheduled DNA synthesis (UDS) test. Nitisinone was mutagenic in the mouse lymphoma cell (L5178Y/TK+/-) forward mutation test and in an in vivo mouse bone marrow micronucleus test.
In a single dose-group study in rats given 100 mg/kg (16.2 times the recommended initial dose of 1 mg/kg/day on a body surface area basis), reduced litter size, decreased pup weight at birth, and decreased survival of pups after birth was demonstrated.
-
14 CLINICAL STUDIES
The efficacy and safety of nitisinone in patients with HT-1 was evaluated in one open-label, uncontrolled study of 207 patients with HT-1, ages 0 to 22 years at enrollment (median age 9 months). Patients were diagnosed with HT-1 by the presence of succinylacetone in the urine or plasma. All patients were treated with nitisinone at a starting dose of 0.3 to 0.5 mg/kg twice daily, and the dose was increased in some patients to 1 mg/kg twice daily based on weight, liver and kidney function tests, platelet count, serum amino acids, urinary phenolic acid, plasma and urine succinylacetone, erythrocyte PBG-synthase, and urine 5-ALA. The median duration of treatment was 22 months (range less than 1 month to 80 months). Efficacy was assessed by comparison of survival and incidence of liver transplant to historical controls.
For patients presenting with HT-1 younger than 2 months of age who were treated with dietary restriction and nitisinone, 2-and 4-year survival probabilities were 88% and 88%, respectively. Data from historical controls showed that patients presenting with HT-1 at younger than 2 months of age and treated with dietary restriction alone had 2-and 4-year survival probabilities of 29% and 29%, respectively. For patients presenting with HT-1 between 2 months and 6 months of age who were treated with dietary restriction and nitisinone, 2-and 4-year survival probabilities were 94% and 94%, respectively. Data for historical controls showed that patients presenting with HT-1 between 2 months and 6 months of age treated with dietary restriction alone had 2-and 4-year survival probabilities of 74% and 60%, respectively.
The effects of nitisinone on urine and plasma succinylacetone, porphyrin metabolism, and urinary alpha-1-microglobulin were also assessed in this clinical study.
Porphyria-like crisis were reported in 3 patients (0.3% of cases per year) during the clinical study. This compares to an incidence of 5 to 20% of cases per year expected as part of the natural history of the disorder. An assessment of porphyria-like crises was performed because these events are commonly reported in patients with HT-1 who are not treated with nitisinone.
Urinary alpha-1-microglobulin, a proposed marker of proximal tubular dysfunction, was measured in 100 patients at baseline. The overall median pretreatment level was 4.3 grams/mol creatinine. After one year of treatment in a subgroup of patients (N=100), overall median alpha-1-microglobulin decreased by 1.5 grams/mol creatinine. In patients 24 months of age and younger in whom multiple values were available (N=65), median alpha-1-microglobulin levels decreased from 5.0 to 3.0 grams/mol creatinine (reference value for age less than or equal to 12 grams/mol creatinine). In patients older than 24 months in whom multiple values were available (N=35), median alpha-1-microglobulin levels decreased from 2.8 to 2.0 grams/mol creatinine (reference for age less than or equal to 6 grams/mol creatinine).
The long term effect of nitisinone on hepatic function was not assessed.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Nitisinone Capsules 10 mg for oral administration containing 10 mg of nitisinone, are supplied as follows:
White Opaque Hard gelatin capsule shell Size #3, imprinted with black ink as "010" on cap and "Novitium 10 mg" on body, filled with white to off white powder blend.
NDC 63629-2233-1 Bottles of 60 capsules with child-resistant closure and tamper resistant induction sealing
Store at room temperature between 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELLING INFORMATION
Administration [see Dosage and Administration (2.2)]
Administration of Nitisinone Capsules
- Maintain dietary restriction of tyrosine and phenylalanine when taking nitisinone.
- Capsules: Take at least one hour before, or two hours after a meal. For patients who have difficulty swallowing the capsules, the capsules may be opened and the contents suspended in a small amount of water, formula or apple sauce immediately before use.
Elevated Plasma Tyrosine Levels, Ocular Symptoms, Developmental Delay and Hyperkeratotic Plaques
- Inform patients that inadequate restriction may be associated with ocular signs and symptoms, intellectual disability and developmental delay, and painful hyperkeratotic plaques on the soles and palms. Advise patients and caregivers of the need to maintain dietary restriction of tyrosine and phenylalanine and to report any unexplained ocular, neurologic, or other symptoms promptly to their healthcare provider [see Warnings and Precautions (5.1)].
Manufactured by:
Novitium Pharma LLC
70 Lake Drive, East Windsor
New Jersey 08520Distributed by:
Par Pharmaceutical
Chestnut Ridge, NY 10977Revised: August, 2019
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NITISINONE
nitisinone capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-2233(NDC:0254-3022) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITISINONE (UNII: K5BN214699) (NITISINONE - UNII:K5BN214699) NITISINONE 10 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) HYPROMELLOSE 2910 (5 MPA.S) (UNII: R75537T0T4) MANNITOL (UNII: 3OWL53L36A) STEARIC ACID (UNII: 4ELV7Z65AP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 16mm Flavor Imprint Code 010;Novitium10mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-2233-1 1 in 1 CARTON 03/22/2021 1 60 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211041 10/04/2019 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-2233) , RELABEL(63629-2233)