Label: KETOMED- ketoprofen injection, solution
- NDC Code(s): 61133-4007-1, 61133-4007-2
- Packager: Bimeda, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
Drug Label Information
Updated January 6, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
DESCRIPTION
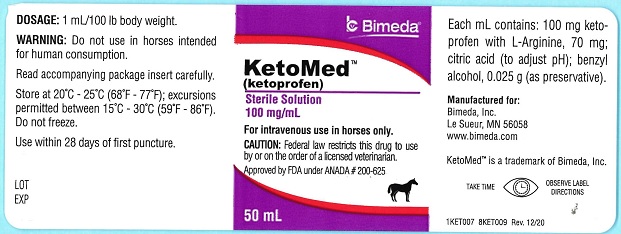
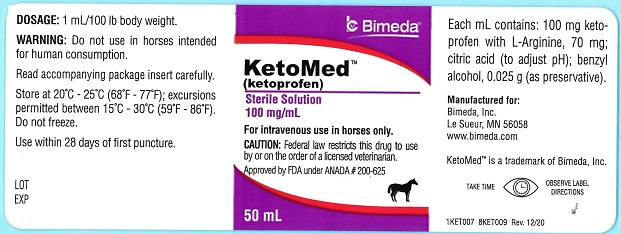
Ketoprofen is a non-steroidal anti-inflammatory agent of the propionic acid class that includes ibuprofen, naproxen, and fenoprofen. Each mL of KetoMed (ketoprofen) contains 100 mg of ketoprofen in an aqueous formulation containing: L-Arginine, 70 mg; citric acid (to adjust pH); benzyl alcohol, 0.025 g (as preservative).
It is packaged in a multiple dose bottle. -
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
PHARMACOLOGY
KetoMed is a non-narcotic, non-steroidal anti-inflammatory agent with analgesic and antipyretic properties.
In horses, intravenous dosages of ketoprofen ranging from 0.5 to 1.5 mg/lb resulted in dosage dependent anti-inflammatory effects in the chronic adjuvant carpitis model as depicted in the following graph.MAXIMUM FLEXION
(intravenous ketoprofen, mean ± sem, n = 4)*
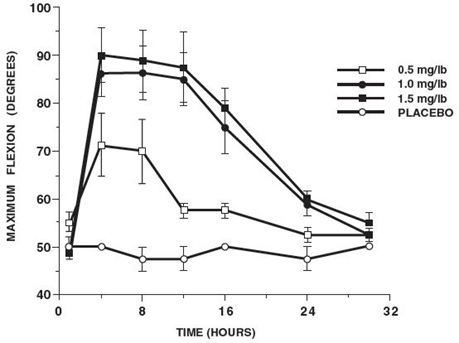
*sem = standard error of the mean
n = number of animals
Additional studies using the same model in horses have shown that the effects of ketoprofen are maximal by 12 hours and still measurable at 24 hours after each dosage as depicted in the following graph.
MAXIMUM FLEXION
(mean ± sem, n = 6)*
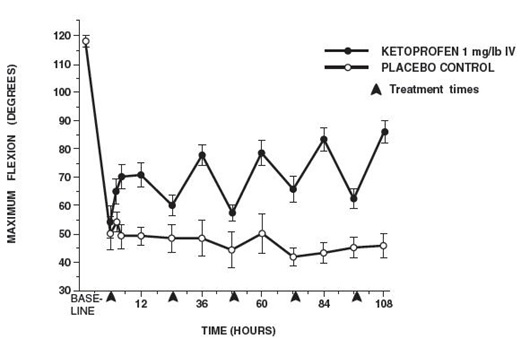
*sem = standard error of the mean
n = number of animals
TOXICITY
Horses were found to tolerate ketoprofen given intravenously at dosages of 0, 1, 3 and 5 mg/lb once daily for 15 consecutive days (up to five times the recommended dosage for three times the usual duration) with no evidence of toxic effects. In clinical studies, intravenous injection of 1 mg/lb/day for five days resulted in no injection site irritation or other side effects.
At 15-fold overdose (15 mg/lb/day) for five days one of two horses developed severe laminitis, but no gross lesions or histologic changes were observed. The toxic effects observed in the horses given a 25-fold overdose (25 mg/lb/day) for five days included inappetence, depression, icterus, abdominal swelling and postmortem findings of gastritis, nephritis and hepatitis.
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- CONTRAINDICATIONS
- SPL UNCLASSIFIED SECTION
- PRECAUTIONS
- WARNINGS
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Bimeda, Inc. at 1-888-524-6332. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KETOMED
ketoprofen injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:61133-4007 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOPROFEN (UNII: 90Y4QC304K) (KETOPROFEN - UNII:90Y4QC304K) KETOPROFEN 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength ARGININE (UNII: 94ZLA3W45F) 70 mg in 1 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61133-4007-1 50 mL in 1 BOTTLE 2 NDC:61133-4007-2 100 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200625 02/08/2021 Labeler - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda- MTC 256232216 manufacture Establishment Name Address ID/FEI Business Operations EUROAPI Germany GmbH 343459891 api manufacture