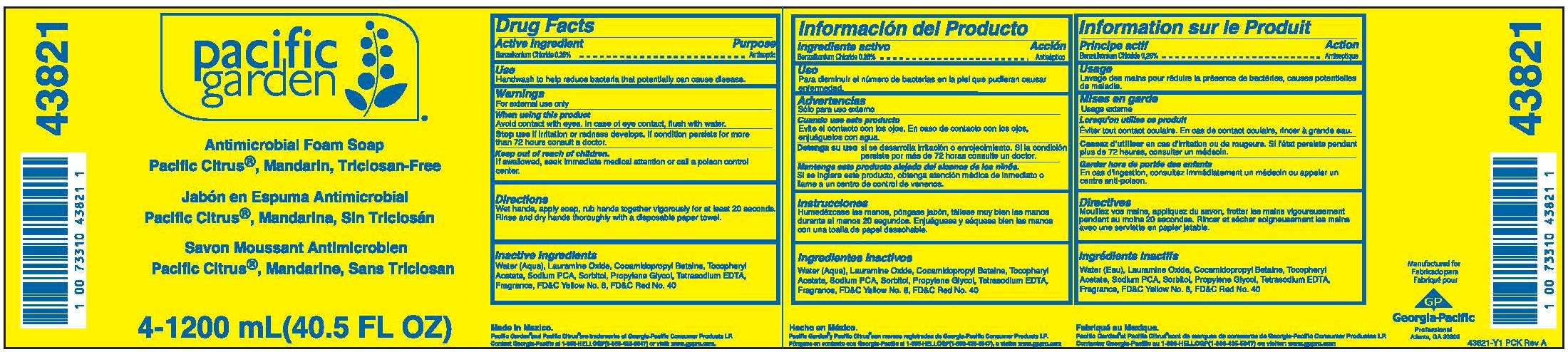
PACIFIC GARDEN ANTIMICROBIAL FOAM PACIFIC CITRUS, MANDARIN, TRICLOSAN-FREE- benzalkonium chloride solution
Georgia-Pacific Consumer Products
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
54622-306
Warnings
- For external use only
Directions
Wet hands, apply soap, rub hands together vigorously for at least 20 seconds.
Rinse and dry hands thoroughly with a disposable paper towel.
| PACIFIC GARDEN ANTIMICROBIAL FOAM PACIFIC CITRUS, MANDARIN, TRICLOSAN-FREE
benzalkonium chloride solution |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Georgia-Pacific Consumer Products (806142217) |
| Registrant - CYAN Labs S.A. de C.V. (812754130) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CYAN Labs S.A. de C.V. | 812754130 | manufacture(54622-306) , label(54622-306) , pack(54622-306) | |
Revised: 12/2022
Document Id: f056f548-b199-eba4-e053-2a95a90a75c1
Set id: c1929b61-37a1-41fb-be78-7d567fa51f0e
Version: 3
Effective Time: 20221221
Georgia-Pacific Consumer Products