Label: GLYCOPYRROLATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 43227-065-01, 43227-066-01 - Packager: Par Formulations Private Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 14, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
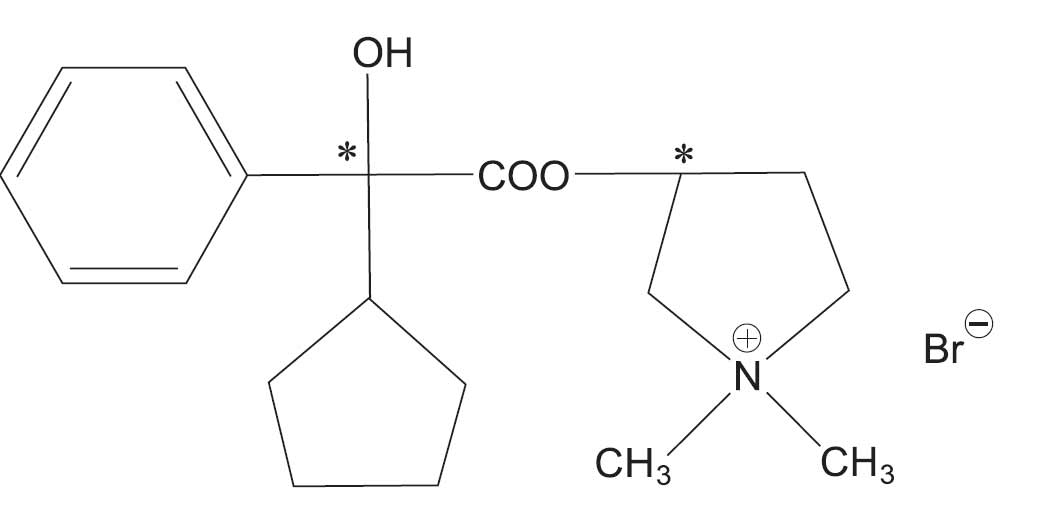
Glycopyrrolate tablets contain the synthetic anticholinergic glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name: 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-
dimethylpyrrolidinium bromide. Its empirical formula is C19H28BrNO3, its molecular weight is 398.33, and its structural formula is:
Each 1 mg tablet contains: Glycopyrrolate, USP ................1 mg
Each 2 mg tablet contains: Glycopyrrolate, USP ................2 mg
Inactive Ingredients: Dibasic Calcium Phosphate, Lactose, Magnesium Stearate, Povidone, Sodium Starch Glycolate.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GLYCOPYRROLATE
glycopyrrolate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43227-065 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOPYRROLATE (UNII: V92SO9WP2I) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRROLATE 1 mg Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONES (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code K;400 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43227-065-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040653 09/26/2006 GLYCOPYRROLATE
glycopyrrolate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43227-066 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOPYRROLATE (UNII: V92SO9WP2I) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRROLATE 2 mg Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONES (UNII: FZ989GH94E) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code K;401 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43227-066-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/26/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040653 09/26/2006 Labeler - Par Formulations Private Limited (676159161) Registrant - Par Formulations Private Limited (676159161)




