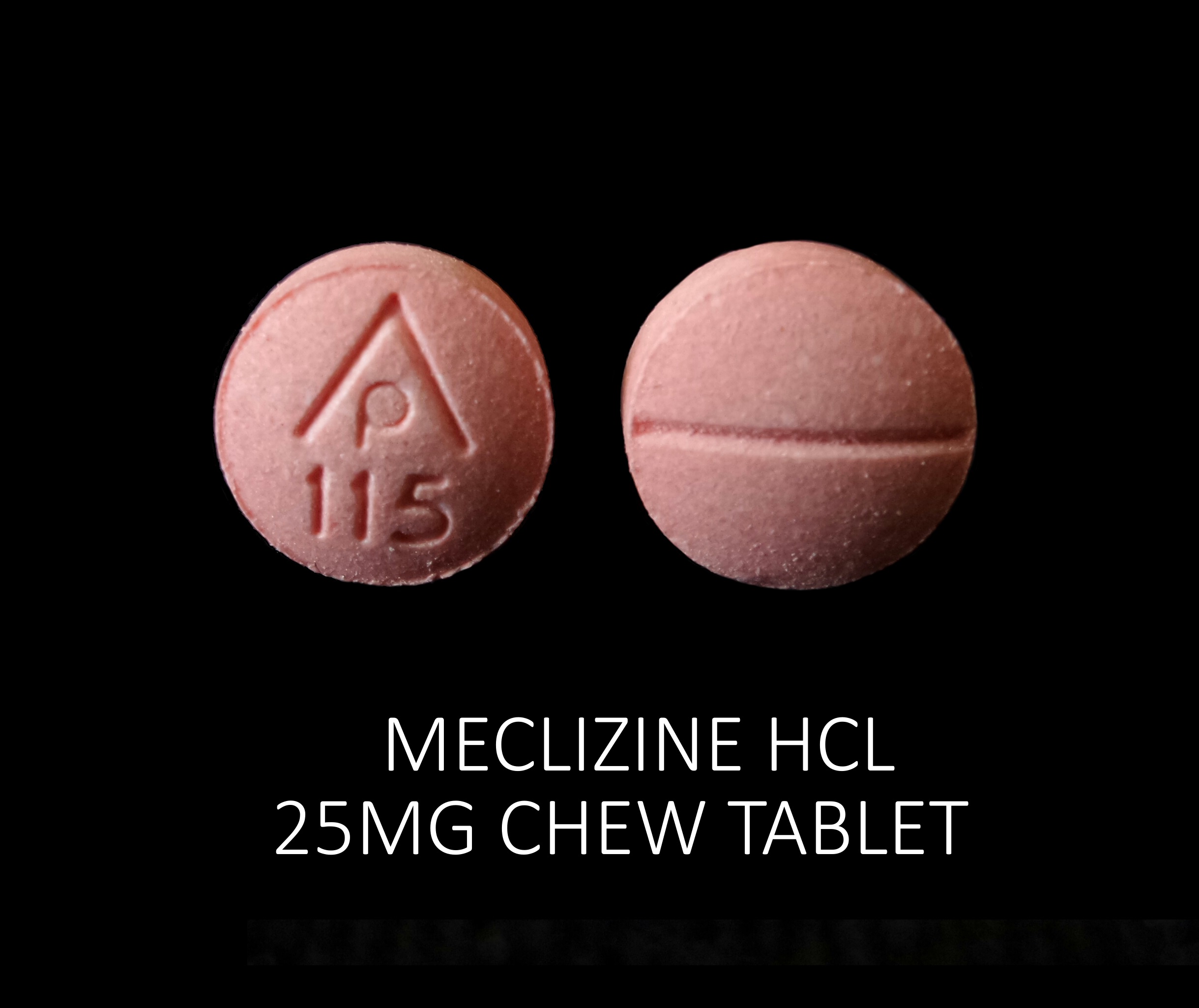
MECLIZINE HCL 25 MG- meclizine hcl tablet
Preferred Pharmaceuticals Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Meclizine HCL 25 mg
Uses
- •
- prevents and treats nausea, vomiting or dizziness due to motion sickness
- •
- for others uses, consult your doctor
Warnings
Ask a doctor before use if you have
- •
- glaucoma
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this prodcut
- •
- you may get drowsy
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Ask a doctor before use if you have
- •
- glaucoma
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- trouble urinating due to an enlarged prostate gland
When using this product
- •
- you may get drowsy
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- •
- take dose one hour before travel starts
- •
- tablets can be chewed or swallowed whole with water
adults & children 12 years and over: 1-2 tablets once daily
children unser 12 years: ask a doctor
Other information
- •
- Phenylketonurics: each tablet contrains: phenylalanine 0.28 mg
- •
- store at room temperature 15°-30°C (59°-86°F)
- •
- This is a bulf package. Dispense contents with a child-resistant closure in a tight, light-resistant container as defined in the USP.
Inactive ingredients
aspartame, compressible sugar, croscarmellose sodium, dextrose, FD&C red # 40 (Al-lake), magnesium stearate, microcrystalline cellulose, raspberry flavor
| MECLIZINE HCL 25 MG
meclizine hcl tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Preferred Pharmaceuticals Inc. (791119022) |
| Registrant - Preferred Pharmaceuticals Inc. (791119022) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Preferred Pharmaceuticals Inc. | 791119022 | REPACK(68788-6451) | |
