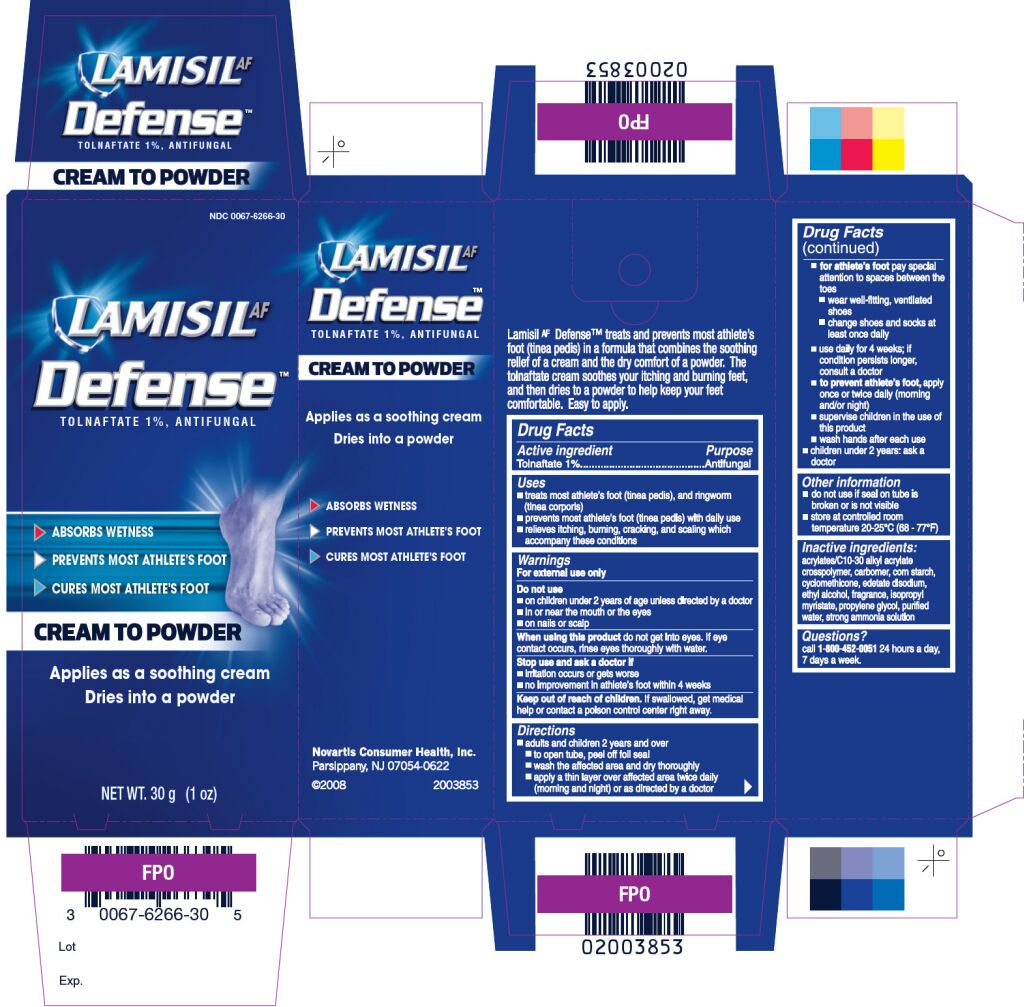
LAMISL AF DEFENSE- tolnaftate cream
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- •
- treats and prevents most athlete’s foot (tinea pedis), and ringworm (tinea corporis)
- •
- prevents most athlete’s foot (tinea pedis) with daily use
- •
- relieves itching, burning, cracking and scaling which accompany these conditions
Do not use
- •
- on children under 2 years of age unless directed by a doctor
- •
- in or near the mouth or the eyes
- •
- on nails or scalp
When using this product
do not get into eyes. If eye contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if
- •
- irritation occurs or gets worse
- •
- no improvement in athlete’s foot within 4 weeks
Keep Out of Reach of Children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- •
- adults and children 2 years of age and over
- •
- to open tube, peel off foil seal
- •
- wash the affected area and dry thoroughly
- •
- apply a thin layer over affected area twice daily (morning and night) or as directed by a doctor
- •
-
for athlete’s foot pay special attention to spaces between the toes
- •
- wear well-fitting, ventilated shoes
- •
- change shoes and socks at least once daily
- •
- use daily for 4 weeks; if condition persists longer, consult a doctor
- •
- to prevent athlete’s foot, apply once or twice daily (morning and/or night)
- •
- supervise children in the use of this product
- •
- wash hands after each use
- •
- children under 2 years of age: as a doctor
Other information
- •
- do not use if seal on tube is broken or is not visible
- •
- store at controlled room temperature 200C- 250C (680F- 770F)
Inactive ingredients
acrylates/C10-30 acrylate cross polymer, carbomer, corn starch, cyclomethicone, edetate disodium, ethyl alcohol, fragrance, isopropyl mysristate, propylene glycol, purified water, strong ammonia solution
| LAMISL AF
DEFENSE
tolnaftate cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GlaxoSmithKline Consumer Healthcare Holdings (US) LLC (079944263) |
Revised: 7/2019
Document Id: 1fc41b26-abd7-4722-b3bd-f65152387f17
Set id: c0ad1ce5-bdb3-43cc-8d07-84f250eb289f
Version: 2
Effective Time: 20190730
GlaxoSmithKline Consumer Healthcare Holdings (US) LLC